肝切除仍是肝肿瘤最重要的治疗方法[1-3]。然而,剩余肝体积(FLR)不足易引起肝功能异常限制了扩大肝切除术。扩大肝切除术后肝功能不足会引起一系列临床症状,例如:术后肝衰竭(PHLF)和小肝综合征(SFSS)。因此,手术成功的关键就在于术后剩余肝脏的体积和功能[4]。为了提高手术切除率,人们先后提出了几个促进肝脏增生的策略,例如:上世纪80年代,Makuuchi等[5]首次将门静脉栓塞(PVE)应用于需要大范围肝切除的患者。PVE或门静脉结扎(PVL)的应用使原来由于剩余肝脏不足而无法手术的患者有了手术的机会。但是PVE或PVL术后肝脏增生需要4~8周的时间才能达到标准,在这期间会造成肿瘤进展而且仍有部分患者会因肝脏增生不足而无法进行肝切除术[6-9]。
2007年德国学者[10]开展了一种新的术式——联合肝脏分割和门静脉结扎的二步肝切除术(ALPPS),随即引起国内外肝胆外科医生的广泛关注[11-12]。ALPPS手术方法的出现解决了PVL或PVE的不足,与PVL或PVE相比ALPPS可以在很短时间内促进剩余肝脏快速增生[13]。但是同时有临床报告[14-15]指出ALPPS术后有很高的并发症率和病死率。目前ALPPS促进肝增生的机制仍不清楚,国内少有动物模型;本实验建立通过对SD大鼠肝脏解剖的学习建立ALPPS动物模型,评价术后肝功能及肝再生情况,可为以后研究其机制、并发症、死因等奠定良好的基础。
1 材料与方法
1.1 实验材料与设计
雄性SD大鼠,体质量200~240 g,由江苏省血吸虫病防治研究所提供,首先用20只SD大鼠进行大鼠肝脏解剖学习及ALPPS手术训练;然后将60只SD大鼠随机均分为PVL组、ALPPS组、假手术组。动物在控制温度、湿度及12 h照明与12 h黑暗交替的环境中喂养,并允许随时自由食用固定标准饲料饮食和清水。实验大鼠实验所用外科手术器械由中国人民解放军第一〇一医院提供;Ki-67抗体购自无锡傲锐东源生物有限公司。
1.2 手术方法
所有大鼠采用10%水合氯醛(3.0~3.5 mL/100 g)腹腔注射麻醉;由正中纵切口入腹腔。PVL组在手术灯下仔细分离出供应肝右叶门静脉支和供应左外叶及中叶左支的门静脉支,分别用4-0丝线结扎,保留肝右中叶分支,将尾状叶切除;ALPPS组除上述步骤外将大鼠肝脏左中叶和右中叶在缺血带处离断,注意缺血带位于肝中叶中静脉和肝左中叶门静脉之间,离断过程中勿伤及肝中叶中静脉。离断肝脏时采用血管钳逐步压榨,膈面的背膜用眼科剪分离,离断过程中采用压迫和4-0丝线结扎的方法止血,最后将止血海绵放入断面以便止血和防止术后断面粘连。假手术组仅游离出门静脉分支,不做处理即关腹。被结扎部分约占全肝重量的70%,切除尾状叶约占10%,术后仔细检查无活动性出血,用3-0丝线关腹。定时观察3组动物的生存情况。术后3组动物继续饲养,并允许随时自由食用固定标准饲料饮食和清水。
1.3 观察指标
3组分别于术后1、2、4、7 d各取材5只,计算各时点大鼠右中叶肝再生率(hepatic regeneration rate,HRR):HRR=(WA-WI)/WI×100%其中WA代表各时间点取标本时肝右中叶的质量(经过解剖12只体质量为200~240 g正常SD雄性大鼠,计算肝右中叶的质量WI约为体质量的0.68%)。抽取大鼠下腔静脉血液4 mL,3 000 r/min 5 min分离血清-80 ℃保存,以备检测各时点谷草转氨酶(AST)、谷丙转氨酶(ALT)、白蛋白(ALB)、总胆红素(TBIL);留取左右肝中叶标本,10%甲醛固定,HE染色,观察肝脏病理情况,计算肝左中叶组织坏死面积:在光学显微镜下(×100)随即选取10个视野拍照留存,然后用Photoshop CS6计算每个照片内肝组织坏死面积,以坏死面积/整个视野面积×100%进行评分[16]。0分:无坏死;1分:坏死面积<25%;2分:25%≤坏死面积<50;3分:50%≤坏死面积<75%;4分:坏死面积≥75%。免疫组织化学检测各时点各组肝右中叶Ki-67水平,高倍视野内(×400)计算肝细胞数及Ki-67阳性细胞数,每张切片随机选取5个视野,视野内Ki-67染色阳性细胞数的百分比即为Ki-67标记率。
1.4 统计学处理
数据采用均数±标准差(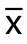 ±s)表示。用SPSS 19.0采用单样本Kolmogorov-Smirnov检验正态,单因素方差分析进行多样本均数比较(组内两两比较采用SNK-q检验)及独立样本t检验进行统计分析,P<0.05为差异有统计学意义。
±s)表示。用SPSS 19.0采用单样本Kolmogorov-Smirnov检验正态,单因素方差分析进行多样本均数比较(组内两两比较采用SNK-q检验)及独立样本t检验进行统计分析,P<0.05为差异有统计学意义。
2 结 果
2.1 手术训练结果
经过20只大鼠的手术训练,掌握了大鼠肝脏的解剖:大鼠肝脏中叶有左右2支门静脉分支和左中右3支肝静脉,选择性PVL后,肝右中叶和左中叶之间出现缺血带,此缺血带位于肝中叶左静脉和中静脉之间,也位于肝中叶门静脉左支和肝中静脉之间,此间隙很窄,更困难是肝脏劈离时要刚好达到肝中静脉,经过学习和实践,笔者发现大概保持与下腔静脉6 mm的距离可以避免伤及肝中静脉而大量出血。手术分3步:⑴ 选择性PVL;⑵ 尾状叶的切除;⑶ 肝中叶的劈断(图1)。
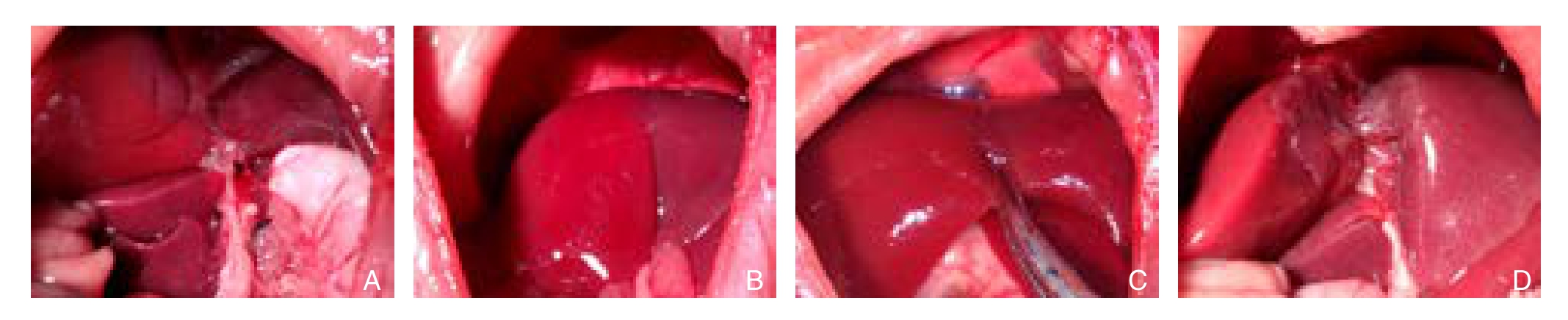
图1 ALPPS手术过程 A:进行肝右叶、左外叶、左中叶门静脉结扎以及尾状叶的切除;B:选择性PVL后出现缺血带;C:用蚊式钳沿着缺血带逐步进行肝脏劈离;D:劈离后的肝脏
Figure 1 Surgical procedure of ALPPS A: Ligation of the portal vein branches for the left lateral, left middle, and right lobes of the liver and caudate lobe resection; B: Present of ischemic boundary after selective PVL; C: Liver transection along the ischemic boundary using mosquito clamp; D: Liver after transection
2.2 肝再生率结果分析
造模实验中的大鼠均存活,无并发症。PVL和ALPPS组各时间点右中叶HRR均明显高于假手术组(均P<0.05),在第4、7天ALPPS组右中叶HRR明显高于PVL组,分别为(155.96±24.39)% vs.(118.15±9.77)%、(174.86±8.99)% vs.(133.55±16.48)%,差异有统计学意义(均P<0.05);第2天,ALPPS组肝再生率高于PVL组,但无统计学差异(P>0.05);第1天两组肝再生率无统计学差异(P>0.05)(图2)。
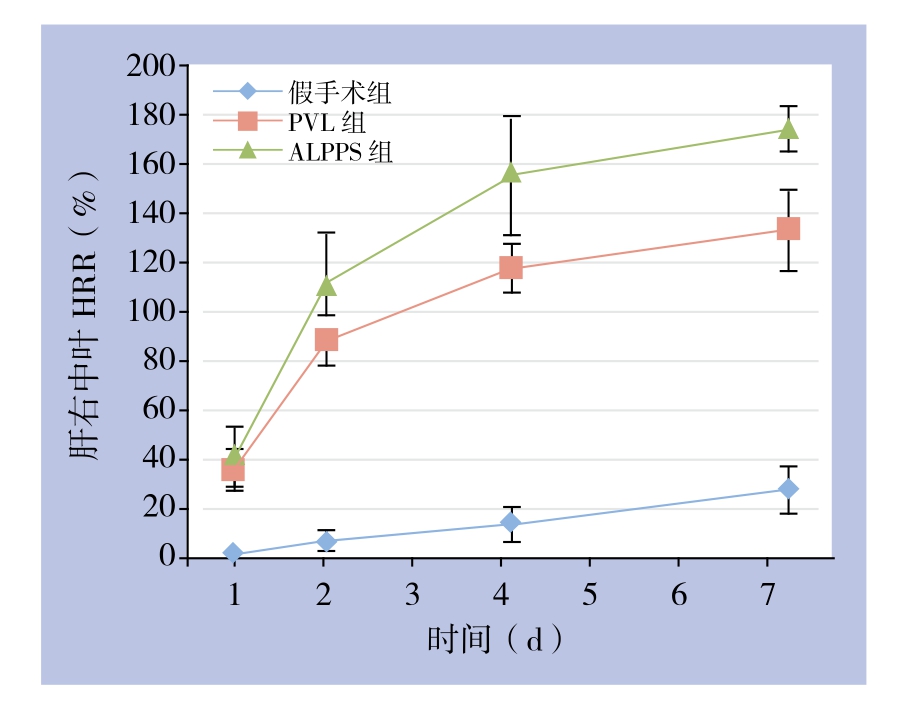
图2 各组术后各时间点肝右中叶HRR
Figure 2 HRR of each group at each postoperative time point after operation
图3 各组各时间点肝功能指标检测情况
Figure 3 Liver function parameters in each group at each postoperative time point
2.3 肝功能检测结果分析
在术后第1、2天ALPPS组和PVL组AST、ALT均明显高于假手术组(均P<0.05),第4、7天组间均无统计学差异(均P>0.05);术后第1天ALPPS组AST、ALT比PVL组明显升高,分别为(2033.40±358.88)U/L vs.(1343.40±230.75)U/L、(982.60±165.63)U/L vs.(410.80±130.08)U/L,差异均有统计学意义(均P<0.05);余时间点未见明显差别(均P>0.05);两组肝功能都在第4天恢复。与假手术组比较,ALPPS组和PVL组的血ALB各时间点均较低(均P<0.05),ALPPS组在第1、2天较PVL组低,分别为(26.18±2.34)g/L vs.(29.90±2.21)g/L、(25.26±2.37)g/L vs.(29.66±2.40)g/L(均P<0.05);术后第4,7天两组差异无统计学意义(均P>0.05);3组TBIL术后各时间点均无统计学差异(均P>0.05)(图3)。
2.4 病理切片结果
HE染色切片观察,假手术组肝左中叶无坏死,PVL组术后第1天肝左中叶坏死面积得分为1.6±0.70,而ALPPS组得分为3.5±0.71,差异有统计学意义(P<0.05)(图4)。
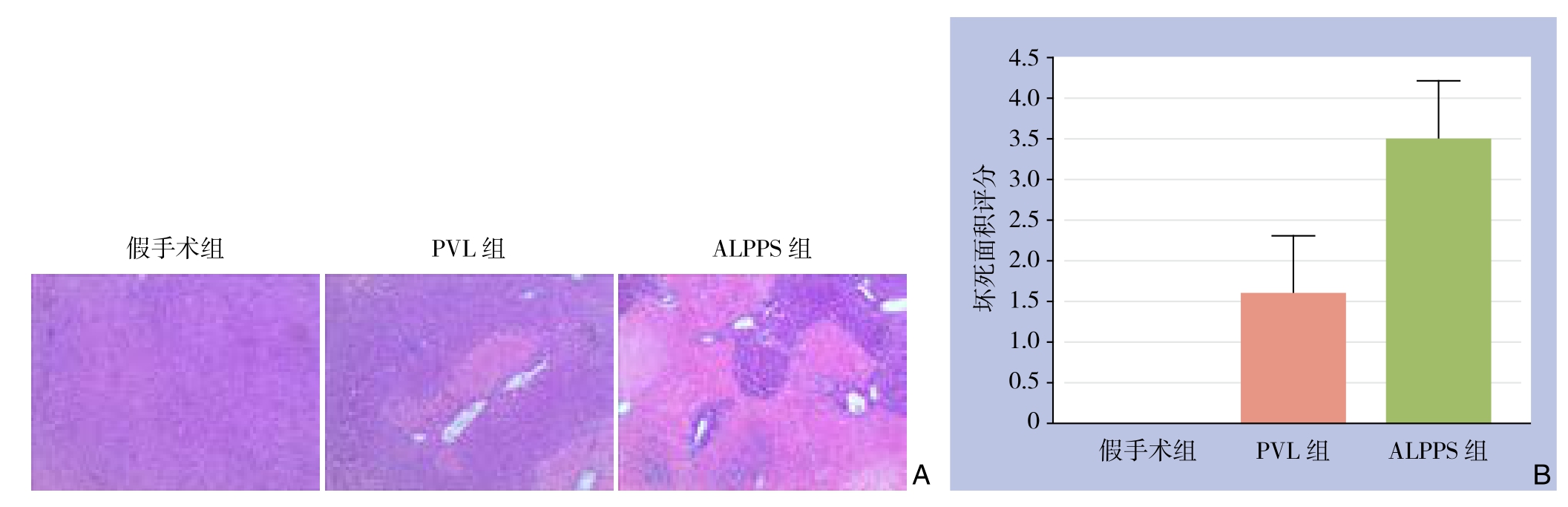
图4 术后第1天肝左中叶坏死情况 A:HE染色(×100);B:坏死面积比较
Figure 4 Necrosis in left middle lobe of the liver on postoperative day 1 A: HE staining (×100); B: Compariosn of the necrosis areas
2.5 Ki-67检测结果
与PVL组比较,ALPPS组Ki-67阳性率在第2、4天明显较高,分别为(85.36±9.13)% vs.(61.84±10.14)%、(43.40±7.89)% vs.(29.06±6.38)%,差异均有统计学意义(均P<0.05),余时间点均无统计学差异(均P>0.05),第7天Ki-67阳性率两组均很低(图5)。

图5 右中叶Ki-67检测 A:Ki-67免疫组化染色(×400);B:ALPPS和PVL术后Ki-67阳性率变化
Figure 5 Determination of Ki-67 in the right middle lobe of the liver A: Immunohistochemical staining for Ki-67 (×400); B: Changes in Ki-67 positive rates in ALPPS group and PVL group after operation
3 讨 论
剩余肝脏体积不足是肝脏巨大肿瘤切除最重要的限制因素。对于正常的肝脏来说,剩余肝脏体积要达到25%以上可以维持代谢平衡,但对于肝功能不良和早期肝损伤的患者比例要达到40%以上[17]。PVE或PVL使肝切除术得到了进一步发展,肝肿瘤的切除范围得到了扩大。但仍有一些患者出现剩余肝体积不足,PVE或PVL二步行肝切除术时由于时间过长肿瘤会进展。而ALPPS解决了以上问题,它可以在短时间内促进肝脏快速增生,Schnitzbauer等[10]报道术后平均9 d、而de Santibañes等[13]报道术后第7天即实施了第二次大范围肝切除术;从而避免了二次手术时肿瘤的进展。可是ALPPS临床应用中出现较高的并发症率和病死率[14-15],且ALPPS肝脏增生的机制并不明确。肝胆外科医生在临床和基础上不断改进、探索,如绕肝提拉法联合ALPPS的应用[18],腹腔镜下ALPPS的应用[19]。本实验的目的是建立大鼠ALPPS模型,观察术后肝功能变化及剩余肝再生情况,为研究ALPPS肝再生机制、术后并发症及死亡原因奠定良好的基础。选择大鼠作为本研究模型有以下几个原因:大鼠肝脏解剖和功能是以Couinaud描述的人类肝脏为基础[20],肝脏是高度可再生的且每叶的肝实质是相对恒定的[21],可以选择性结扎70%以上门静脉血流从而最快促进肝再生。大鼠的肝右中叶约占大鼠总肝的20%[22],这与临床中PVL或ALPPS的需求相近[23-24]。本研究将大鼠肝脏尾状叶切除,模仿经典肝部分切除,进一步促进肝再生。
本研究结果显示,大鼠的肝中叶可模仿人体肝脏,有2支门静脉和3支肝静脉,经过选择性门静脉结扎后,缺血带会出现在肝中叶左侧和右侧之间,也位于左中叶门静脉和肝中叶中静脉之间,实践证明沿着此缺血带劈离肝脏出血很少,有少量出血可采用压迫和缝扎的方法止血,注意术中勿伤及肝中静脉。与假手术组比较,ALPPS组、PVL组均有效促进了肝再生(均P<0.05),但ALPPS组在术后第4、7天明显比PVL组肝再生率高(均P<0.05),术后第1、2天肝再生率差异不显著。ALPPS和PVL可能有相同的促进肝再生的机制,但是ALPPS有更强的刺激因素;在第2天ALPPS组已有高于PVL的趋势,分析这正在为肝脏快速增生做准备,此时肝脏增生不明显,肝细胞的增殖需要时间。用免疫组化方法检测Ki-67去验证这一结果。Ki-67作为一种增殖细胞核相关抗原,与细胞有丝分裂密切相关,表达范围覆盖除G0期以外各增殖期细胞,可以较好的反应细胞处于增殖状态[25-26]。免疫组化检测ALPPS组Ki-67术后第2、4天明显高于PVL组(均P<0.05);术后7 d的Ki-67表达很低,肝脏增生较少,达到稳定状态。
PVL和ALPPS都可促进肝再生,在1、2天无明显差异,但在第4天肝再生率明显增高,笔者推测:⑴ 可能与肝脏血流阻断有关,肝脏劈离后,左中叶血流减少且萎缩坏死更加严重,肝脏丢失更多,从而刺激肝脏增生[22];⑵ 肝中叶离断引起引起炎症反应,释放炎症因子(如IL-6、TNF-α等)刺激肝脏再生[27]。肝左中叶劈离后1天,病理显示坏死面积明显比ALPPS组大,血AST、ALT第1天也比PVL组高,这与劈离后左中叶坏死面积较大相符。ALB检测也发现ALPPS组比PVL组低,这可能与肝损伤、应激、左中叶的坏死相关。这些因素有可能在一定程度上促进了肝脏再生;同时这些因素也可能是引起ALPPS并发症和死亡率高的原因,这有待进一步实验探讨。
综上所述,本实验成功建立了大鼠ALPPS模型,验证了其可在较短时间促进肝再生并探讨了其可能增生机制。本实验存在一定的缺陷:大鼠模型和人类有差别,实验所用大鼠肝脏均是正常肝脏,肝肿瘤时一般会肝硬化或肝功能不良;但本实验可以为进一步研究ALPPS肝再生的机制、相关并发症、病死率以及肝硬化、肝肿瘤做基础。
参考文献
[1] Troja A, Khatib-Chahidi K, El-Sourani N, et al. ALPPS and similar resection procedures in treating extensive hepatic metastases:our own experiences and critical discussion[J]. Int J Surg, 2014,12(9):1020–1022. doi: 10.1016/j.ijsu.2014.07.006.
[2] Abulkhir A, Limongelli P, Healey AJ, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis[J]. Ann Surg, 2008, 247(1):49–57.
[3] Hwang M, Jayakrishnan TT, Green DE, et al. Systematic review of outcomes of patients undergoing resection for colorectal liver metastases in the setting of extra hepatic disease[J]. Eur J Cancer,2014, 50(10):1747–1757. doi: 10.1016/j.ejca.2014.03.277.
[4] Clavien PA, Petrowsky H, DeOliveira ML, et al. Strategies for safer liver surgery and partial liver transplantation[J]. N Engl J Med,2007, 356(15):1545–1559.
[5] Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report[J]. Surgery, 1990, 107(5):521–527.
[6] Wilms C, Mueller L, Lenk C, et al. Comparative study of portal vein embolization versus portal vein ligation for induction of hypertrophy of the future liver remnant using a mini-pig model[J]. Ann Surg,2008, 247(5):825–834. doi: 10.1097/SLA.0b013e31816a9d7c.
[7] Broering DC, Hillert C, Krupski G, et al. Portal vein embolization vs. portal vein ligation for induction of hypertrophy of the future liver remnant[J]. J Gastrointest Surg, 2002, 6(6):905–913.
[8] Kokudo N, Tada K, Seki M, et al. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization[J]. Hepatology, 2001, 34(2):267–272.
[9] Azoulay D, Castaing D, Smail A, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization[J]. Ann Surg, 2000, 231(4):480–486.
[10] Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings[J]. Ann Surg, 2012, 255(3):405–414. doi: 10.1097/SLA.0b013e31824856f5.
[11] 周俭, 王征, 孙健, 等. 联合肝脏离断和门静脉结扎的二步肝切除术[J]. 中华消化外科杂志, 2013, 12(7):485–489. doi:10.3760/cma.j.issn.1673–9752.2013.07.002.Zhou J, Wang Z, Sun J, et al. Associating liver partition and portal vien ligation for staged hepatectomy[J]. Chinese Journal of Digestive Surgery, 2013, 12(7):485–489. doi:10.3760/cma.j.issn.1673–9752.2013.07.002.
[12] 王志明, 陶一明, 黄云, 等. 联合肝脏离断和门静脉切断二步肝切除术在肝炎后肝硬化肝癌中的应用[J]. 中国普通外科杂志,2014, 23(7):867–872. doi:10.7659/j.issn.1005–6947.2014.07.001.Wang ZM, Tao YM, Huang Y, et al. Associating liver partition and portal vein ligation for staged hepatectomy procedure in treatment of hepatocellular carcinoma with post-hepatitic cirrhosis[J]. Chinese Journal of General Surgery, 2014, 23(7):867–872. doi:10.7659/j.issn.1005–6947.2014.07.001.
[13] de Santibañes E, Clavien PA. Playing Play-Doh to prevent postoperative liver failure: the "ALPPS" approach[J]. Ann Surg,2012, 255(3):415–417. doi: 10.1097/SLA.0b013e318248577d.
[14] Schadde E, Ardiles V, Slankamenac K, et al. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies:results of a multicenter analysis[J]. World J Surg, 2014, 38(6):1510–1519. doi: 10.1007/s00268–014-2513–3.
[15] Schadde E, Ardiles V, Robles-Campos R, et al. Early survival and safety of ALPPS: first report of the International ALPPS Registry[J]. Ann Surg, 2014, 260(5):829–836. doi: 10.1097/SLA.0000000000000947.
[16] Veteläinen R, Dinant S, van Vliet A, et al. Portal vein ligation is as effective as sequential portal vein and hepatic artery ligation in inducing contralateral liver hypertrophy in a rat model[J]. J Vasc Interv Radiol, 2006, 17(7):1181–1188.
[17] Tanaka K, Shimada H, Matsuo K, et al. Regeneration after twostage hepatectomy vs. repeat resection for colorectal metastasis recurrence[J]. J Gastrointest Surg, 2007, 11(9):1154–1161.
[18] 王志明, 胡宽, 苌群刚, 等. 绕肝提拉法在联合肝脏离断和门静脉切断二步肝切除术中的应用[J]. 中国普通外科杂志, 2015,24(1):12–17. doi:10.3978/j.issn.1005–6947.2015.01.003.Wang ZM, Hu K, Chang QG, et al. Application of liver hanging maneuver in associating liver partition and portal vein ligation for staged hepatectomy[J]. Chinese Journal of General Surgery, 2015,24(1):12–17. doi:10.3978/j.issn.1005–6947.2015.01.003.
[19] Xiao L, Li JW, Zheng SG. Totally laparoscopic ALPPS in the treatment of cirrhotic hepatocellular carcinoma[J]. Surg Endosc,2015, 29(9):2800–2801. doi: 10.1007/s00464–014-4000–1.
[20] Aller MA, Arias N, Prieto I, et al. A half century (1961–2011) of applying microsurgery to experimental liver research[J]. World J Hepatol, 2012, 4(7):199–208. doi: 10.4254/wjh.v4.i7.199.
[21] Madrahimov N, Dirsch O, Broelsch C, et al. Marginal hepatectomy in the rat: from anatomy to surgery[J]. Ann Surg, 2006, 244(1):89–98.
[22] Wei W, Zhang T, Zafarnia S, et al. Establishment of a rat model:Associating liver partition with portal vein ligation for staged hepatectomy[J]. Surgery, 2016, 159(5):1299–307. doi: 10.1016/j.surg.2015.12.005.
[23] Abdalla EK. Portal vein embolization (prior to major hepatectomy)effects on regeneration, resectability, and outcome[J]. J Surg Oncol,2010, 102(8):960–967. doi: 10.1002/jso.21654.
[24] Ribero D, Curley SA, Imamura H, et al. Selection for resection of hepatocellular carcinoma and surgical strategy: indications for resection, evaluation of liver function, portal vein embolization, and resection[J]. Ann Surg Oncol, 2008, 15(4):986–992. doi: 10.1245/s10434–007-9731-y.
[25] Guinebretière JM, Sabourin JC. Ki-67, marker of proliferation[J].Ann Pathol, 1997, 17(1):25–30.
[26] Duchrow M, Schlüter C, Key G, et al. Cell proliferation-associated nuclear antigen defined by antibody Ki-67: a new kind of cell cyclemaintaining proteins[J]. Arch Immunol Ther Exp (Warsz), 1995,43(2):117–121.
[27] Schlegel A, Lesurtel M, Melloul E, et al. ALPPS: from human to mice highlighting accelerated and novel mechanisms of liver regeneration[J]. Ann Surg, 2014, 260(5):839–846. doi: 10.1097/SLA.0000000000000949.
