乳腺癌是女性最常见的恶性肿瘤之一,病死率高。乳腺癌的筛查对其早期诊断至关重要,Siegel等[1]报道乳腺癌首次诊断时的分期与5年生存率关系密切,乳腺癌I期的5年生存率可达到100%,而到了IV期生存率仅约5%。目前,乳腺癌主要的筛查技术有X线钼靶检查、超声、MRI等,但X线钼靶检查的整体特异性不高,且有65%~90%的假阳性率,尤其是对于部分乳腺腺体致密的女性[2]。吕涛等[3]研究表明绝经前女性、乳腺腺体致密的女性超声检查比X线钼靶更易检出早期乳腺肿块。乳腺超声也可以与钼靶相结合,互相补充,减少需要活检的患者数量[4]。彭友等[5]探讨了超声联合钼靶定位活检乳腺微钙化灶的临床应用价值,结果表明两者联合定位对微钙化病灶定位更精准。MRI则价格昂贵,检查时间长,难以推广应用。超声检查由于对患者无痛苦、无损伤、无辐射,价格便宜,可反复进行等优势,成了乳腺疾病检查的主要手段,在乳腺癌筛查中发挥着非常重要的临床作用[6]。但乳腺癌在声像图上表现的多样性,使得临床早期明确诊断和评估预测需要更多的信息,因此多模态超声新技术的联合应用对乳腺癌的诊断尤为重要[7]。乳腺多模态超声相关的新兴技术,包括乳腺复合成像,自动乳腺全容积成像,超声弹性成像,乳腺超声造影等技术,这些新技术在临床实践中应用广泛,并在将来有更广阔的应用前景。
1 乳腺复合成像(breast compound imaging)
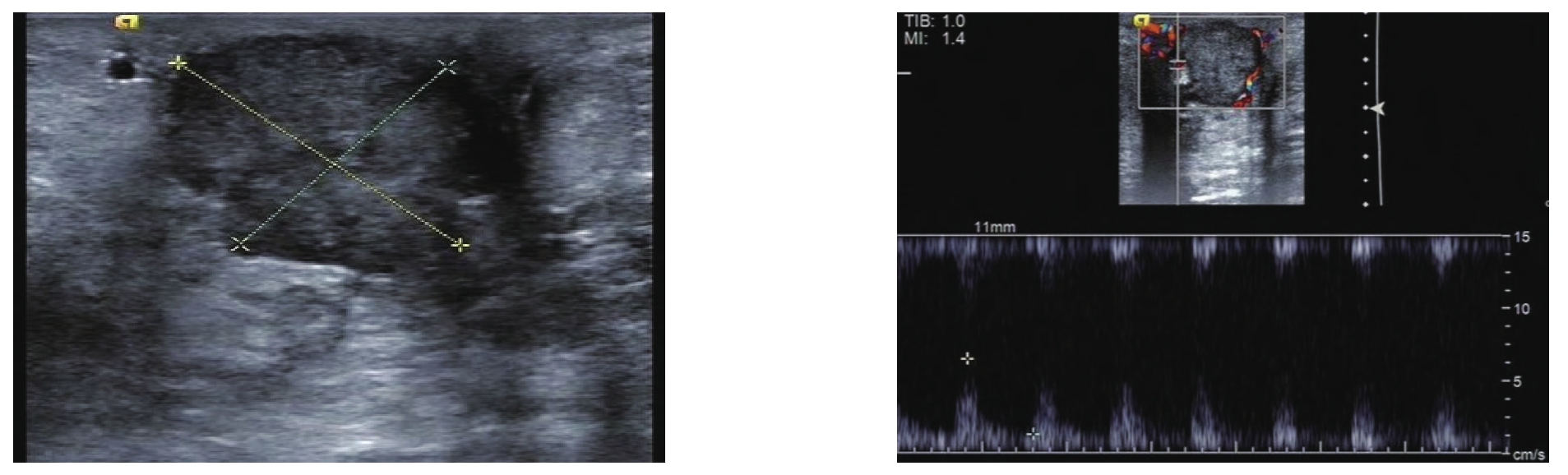
图1 乳腺复合成像
Figure 1 Breast compound imaging
乳腺复合成像技术包括空间复合成像和频率复合成像。空间复合成像指从不同声束方向获得的超声图像组成一个复合图像,与常规图像相比,能提高分辨率,可清晰显示组织的边界,增加低对比度组织及微小病灶的显示率,有更好的整体图像质量。频率复合成像指使用多个滤波器将从不同的频段获得的图像组合成一个复合图像,可以抑制斑点噪声,增加对比分辨率及提高穿透力。空间复合图像的质量与组织的深度有关,而频率复合不易受深度的影响[8-9](图1)。杨秋娟等[10]应用常规超声和超声复合成像技术诊断早期乳腺癌患者,与病理学结果对比发现,超声复合成像技术诊断准确性较常规超声明显增加,可显著提高乳腺癌的早期诊断率。
2 组织谐波成像(tissue harmonic imaging,THI)
THI指通过优化非线性组织信号组件的接收过滤器,使扫描仪只接受两倍于发射频率的谐波,对其进行放大成像。能减少杂乱回声,降低伪像,提高图像的信噪比[11-12]。THI提高了乳腺病灶与腺体及脂肪组织之间的灰阶对比度[13],更适用于肥胖患者的乳腺检查。与二维基波成像相比,THI能更清晰地显示病灶内部的微小钙化灶,且可显著提高乳腺病灶的边界显示率,王广珊等[14]发现仅使用二维基波成像完成乳腺超声的检查是不足够的,需联合使用THI技术以提高乳腺病灶与周边正常组织的对比分辨力,以更好的观察病灶的内部回声及周边情况。Hooley等[9]发现联合使用空间复合成像和THI,可提高图像的整体质量,增加病灶的显示率,提高诊断准确性。
3 萤火虫技术(micropure system technology)
萤火虫技术是一种新型超声图像处理技术,可后处理灰阶超声图像,以提高乳腺微钙化的可视性。该技术使用滤波器,将每个像素与周围区域的平均亮度进行对比,以检测出与周围区域有特征性变化的部位。由于萤火虫成像技术的焦点是检测乳腺组织中的微钙化,所以在水平方向上优化滤波核,以仅检测与周围乳腺组织相比具有更高亮度的孤立点[15]。Machado等[16]研究表明,萤火虫技术可以检测到比常规超声更多的微钙化。但是,这种方法并不适合筛查,它的灵敏度不及X线钼靶检查,但可用于引导活检等更有针对性的应用。
4 自动乳腺全容积成像(automated breast volume scanner,ABVS)
ABVS系统是一种新的三维容积超声成像系统,与传统超声技术扫查乳腺肿块比较,其优势在于可获得整个乳腺连续的横切面数据,并可自动进行三维重建,同步获得整个乳腺包括矢状面和冠状面的图像,从而充分显示乳腺肿块与周围组织的关系,获得肿瘤生长和侵袭改变等信息[17-18],冠状面所示的“汇聚征”是乳腺癌的特征性改变(图2)。朱罗茜等[19]比较了ABVS与常规超声检查鉴别诊断BI-RADS4类肿块良恶性的价值,结果显示ABVS鉴别BI-RADS4 类乳腺肿块良恶性的敏感度、特异度、准确性和曲线下面积均高于常规超声,表明ABVS能更早检查出乳腺癌。Chae等[20]将联合使用X线钼靶和ABVS与单独使用X线钼靶进行了对比,结果表明,每1 000例高危女性中额外有2.391例被查出患有乳腺癌,这表明ABVS能检测出微小的病灶,提高检出率。ABVS也可用来评估乳腺癌患者的化疗疗效,Wang等[21]通过ABVS分别获得行辅助化疗前后肿块的大小及声像图特征等信息,比较其变化以评估化疗的疗效,发现ABVS是早期评估新辅助化疗后病理完全缓释病例的重要工具。ABVS在乳腺癌的筛查、诊断,以及乳腺癌患者的随访中,都有非常重要的意义。但ABVS也有其局限性,如不能显示肿块的血流分布情况,对微钙化的显示也欠佳,且无法扫查腋窝组织,所以必须联合手持式超声进行检查,必要时辅以其他检查方式。
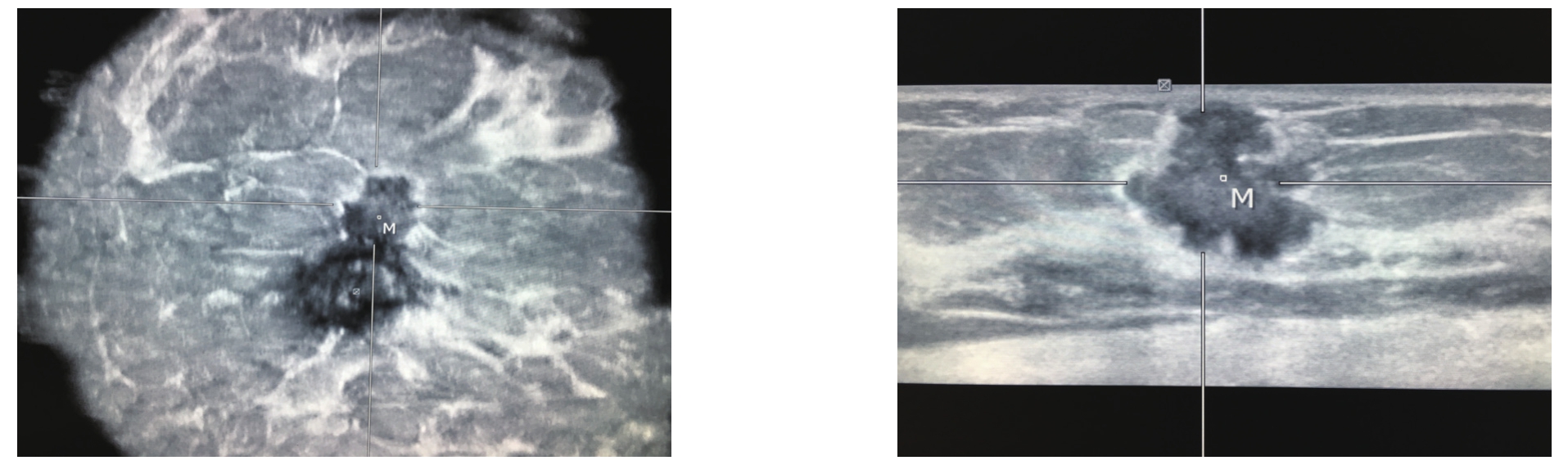
图2 ABVS
Figure 2 ABVS
5 弹性成像(elasticity imaging)
利用超声成像方法,结合数字信号处理或数字图像处理技术,估计出组织内部的相应变化,间接或直接反映组织内部的弹性模量等力学属性的差异即超声弹性成像。常用的弹性成像技术主要包括应变弹性成像(SE)和剪切波弹性成像(SWE)[22](图3)。乳腺内不同组织的弹性值不一样,从小到大依次为脂肪及腺体、纤维腺瘤、导管内乳头状瘤、腺病、浸润性导管癌[23]。2015年,世界医学和生物学超声联合会(WFUMB)公布了最新的弹性成像临床使用指南,推荐使用弹性成像来检测乳腺肿块[24]。倪梁红等[25]的研究结果显示弹性成像能很好地显示乳腺肿块及其周边的情况,并可通过超声图像上的不同颜色进行定性评估。赵巧玲等[26]研究了弹性应变率比值鉴别乳腺肿块良恶性的价值,结果表明,弹性成像联合二维超声能提高乳腺肿块的术前诊断符合率。Lee等[27]研究发现SWE对乳腺良恶性肿块的鉴别有意义,恶性病灶SWE值明显大于良性病灶。周建桥等[28]使用弹性成像对120例乳腺癌患者共120个肿块良恶性进行了评估,诊断的灵敏度为69.39%,特异度为91.55%,准确率为82.50%,结论表明弹性成像有助于乳腺良恶性肿块的鉴别诊断。弹性成像评估乳腺肿块良恶性的主要优点是特异性比普通二维超声高,可以区分BI-RADS3类和BIRADS4A类,避免穿刺活检[29-30]。Hayashi等[31]发现乳腺肿块的硬度可能与淋巴结受累情况密切相关,SE或SWE或许也可用于检测恶性病变的转移特性。Hao等[32]提出良性和恶性细胞的微环境是不同的,与恶性相关的侵袭性与肿块的微环境变化相关,会使弹性成像中的硬度值发生变化,这进一步表明弹性成像可用于识别乳腺癌转移。但弹性成像也有其不足之处,对操作者依赖性高,有主观因素干扰,也可能出现误诊的情况,因此在日常工作中应特加注意。
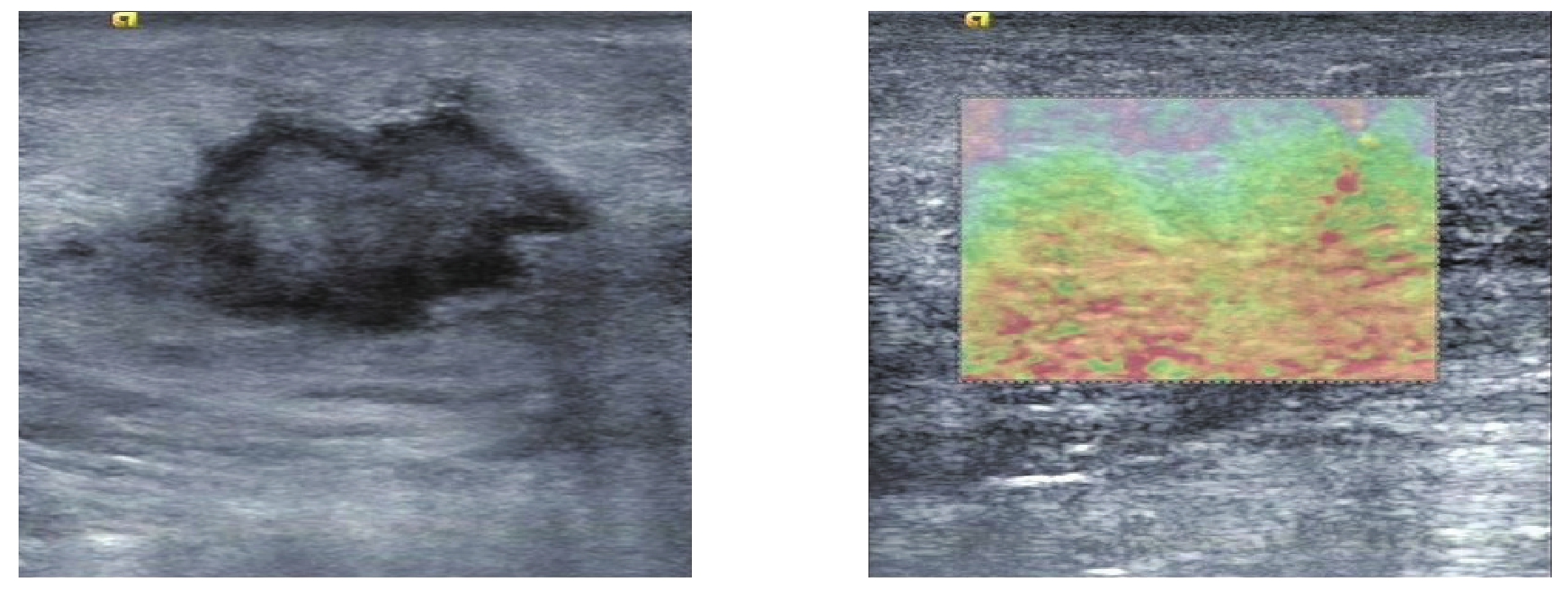
图3 弹性成像
Figure 3 Elasticity imaging
6 超声造影(contrast-enhanced ultrasound,CEUS)
CEUS指利用造影剂来增强血液的后散射回声,观察正常组织与肿块的血流灌注情况,以鉴别肿块的良恶性,提高诊断准确性。超声造影剂由气体微泡组成,气体微泡经外壳密封包装以确保其稳定性。这些微泡通常经静脉注射,它们的大小(<8 μm)使得它们仅局限于在血管内运动[33]。常婉英等[34]探讨了CEUS的边缘特征对乳腺良恶性肿块的鉴别诊断价值,得出了其对乳腺良恶性肿块的鉴别诊断有一定的临床意义的结论。Zhao等[35]探讨了CEUS对乳腺癌的诊断价值及预后价值,得出了CEUS是乳腺癌早期诊断及判断预后的有用工具的结论。王小燕等[36]研究发现恶性肿块超声造影的敏感度、特异度、阳性预测值、阴性预测值均高于其二维超声检查,超声造影可增强对乳腺肿块低速血流的显示,有助于乳腺良恶性病变的鉴别诊断。Luo等[37]对230名患者共235个常规超声分类为BI-RADS4类的乳腺实性肿块进行了评估,以确定CEUS是否可以提高乳腺影像报告和数据系统(BI-RADS)的分类精确度,结果表明用CEUS再次评估BI-RADS 4类乳腺肿块可降低其活检率。杨勇等[38]比较了CEUS与增强磁共振成像鉴别诊断在常规超声中分类为BI-RADS3-4类的乳腺肿块的良恶性的临床应用价值,结果表明CEUS在乳腺良恶性肿块的鉴别诊断中有一定的诊断价值,与增强磁共振具有较高的一致性。常规超声是CEUS的基础,CEUS必须与其相结合,才能发挥作用。目前乳腺CEUS还没有相对统一且被广泛认可的诊断标准,这在某种程度上限制了其在临床上的广泛应用。
7 超声引导细针穿刺(fine needle aspiration cytology,FNAC)
FNAC指利用细针穿刺病灶,获取少许组织做涂片检查的一种细胞学诊断方法,有快速诊断、损伤小且病理符合率较高等优点,为临床上常用的诊断手段(图4)。对于触诊不可扪及或直径<1 cm的乳腺病灶,若盲目穿刺则不易取到理想标本,检出率及准确率降低,而在超声引导定位下穿刺,可以直视病灶的内部结构,并可多点取材,成功率高,增加了细胞学检查的敏感性。超声引导下穿刺优于钼靶X线定位穿刺活检,它避免了放射损伤,对操作者要求也不高[39]。超声引导下的FNAC能提高诊断恶性肿块的准确度,在临床上乳腺良恶性肿块的鉴别诊断中发挥着重要作用,某种程度上甚至可以取代术中的快速冷冻病理切片检查[40],但对于分化好的乳腺恶性肿块,FNAC无法排除有假阳性情况存在时,还是应常规做术中快速冷冻病理切片以明确诊断。Alkuwar等[41]认为超声引导下的FNAC是一种简单的微创技术,还可用于了解乳腺癌患者术前的淋巴结状态,即是否发生了淋巴结转移,以确定外科手术方式,是选择前哨淋巴结清扫术还是全淋巴结清扫术,具有较高的阳性预测值。Barco等[42]等研究了二维彩超、超声引导下的FNAC与MRI单独或联合作用是否能预测腋窝淋巴结受累,结果表明二维彩超的灵敏度与阴性预测值高于MRI,特异度与阳性预测值低于MRI,联合使用两项检查时仅特异度与阳性预测值略有升高,而FNAC的灵敏度为80%,特异度与阳性预测值为100%,得出了腋窝彩超及超声引导下的FNAC必须被包含在乳腺癌患者的术前检查中的结论。但FNAC也具有一定的局限性:由于只获取少许组织,故可能出现假阳性或假阴性,导致误诊,所以对于高度可疑病例,还是应行常规活检或者术中行快速冷冻病理切片证实。
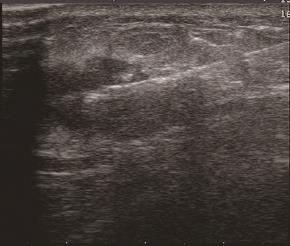
图4 FNAC
Figure 4 FNAC
8 结 论
超声集便携、无电离辐射、分辨率高和成本低等优势于一体,不仅是乳腺癌筛查的主要手段,提高了早期乳腺癌的检出率,在乳腺癌患者的随访中,也有不可替代的作用。随着技术的进步,ABVS、弹性成像、超声造影等新兴多模态超声新技术逐渐为临床所用。在鉴别乳腺肿块良恶性时,应以二维超声为基础,辅以彩色多普勒血流成像,联合应用多模态超声诊断新技术,全面的进行判断分析,提高诊断准确率。
参考文献
[1]Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017[J]. CA Cancer J Clin, 2017, 67(1):7–30. doi: 10.3322/caac.21387.
[2]Weigert J, Steenbergen S. The connecticut experiments second year:ultrasound in the screening of women with dense breasts[J]. Breast J,2015, 21(2):175–180. doi: 10.1111/tbj.12386.
[3]吕涛, 吕晓玉, 汪湍, 等. 超声与钼靶摄片对乳腺癌早期诊断的意义[J]. 中国普通外科杂志, 2013, 22(11):1414–1419. doi:10.7659/j.issn.1005–6947.2013.11.009.Lu T, Lu XY, Wang T, et al. Ultrasound and molybdenum target X-ray mammography for early diagnosis of breast cancer[J].Chinese Journal of General Surgery, 2013, 22(11):1414–1419.doi:10.7659/j.issn.1005–6947. 2013.11.009.
[4]Taylor KJ, Meritt C, Piccoli C, et al. Ultrasound as a complement to mammography and breast examination to characterize breast masses[J]. Ultrasound Med Biol, 2002, 28(1):19–26.
[5]彭友, 罗中尧, 丁金旺, 等. 钼靶联合超声引导双定位法在乳腺微钙化灶活检中的临床应用研究[J]. 中国普通外科杂志, 2016,25(11):1633–1639. doi:10.3978/j.issn.1005–6947.2016.11.019.Peng Y, Luo ZY, Ding JW, et al. Clinical application of combined localization of X-ray mammography and ultrasonography in biopsy of breast microcalci fi cation[J]. Chinese Journal of General Surgery, 2016, 25(11):1633–1639.doi:10.3978/j.issn.1005–6947.2016.11.019.
[6]Wilczek B, Wilczek HE, Rasouliyan L, et al. Adding 3D automatd breast ultrasound to mammography screening in women with heterogeneously and extremely dense breasts: Report from a hospital-based, high-volume, single-center breast cancer screening program[J]. Eur J Radiol, 2016, 85(9):1554–1563. doi: 10.1016/j.ejrad.2016.06.004.
[7]Zografos G, Liakou P, Koulocheri D. Differentiation of BIRADS-4 small breast lesions via Multimodal Ultrasound Tomography[J]. Eur Radiol, 2015, 25(2):410–418. doi: 10.1007/s00330–014–3415–3.
[8]Mesurolle B, Bining HJ, El Khoury M, et al. Contribution of tissue harmonic imaging and frequency compound imaging in interventional breast sonography[J]. J Ultrasound Med, 2006,25(7):845–855.
[9]Hooley R, Scoutt L, Philpotts L. Breast ultrasonography: state of the art[J]. Radiology, 2013, 268(3):642–659. doi: 10.1148/radiol.13121606.
[10]杨秋娟. 超声复合成像技术在早期乳腺癌诊断中的应用体会[J].现代诊断与治疗, 2014, 25(23):5388–5389.Yang QJ. Experience in using ultrasonic compound imaging for diagnosis of early breast cancer[J]. Modern Diagnosis & Treatment,2014, 25(23):5388–5389.
[11]Anvari A, Forsberg F, Samir AE. Primer on the Physical Principles of Tissue Harmonic Imaging[J]. Radiographics, 2015, 35(7):1955–1964. doi: 10.1148/rg.2015140338.
[12]Clevert D, Jung EM, Jungius KP, et al. Value of tissue harmonic imaging (THI) and contrast harmonic imaging (CHI) in detection and characterisation of breast tumours[J]. Eur Radiol, 2007,17(1):1–10.
[13]Cha JH, Moon WK, Cho N, et al. Characterization of benign and malignant Solid breast masses: comparison of conventional US and tissue harmonic imaging[J]. Radiology, 2007, 242(1):63–69.
[14]王广珊, 李志华, 杨世昕. 超声斑点抑制成像联合组织谐波成像在乳腺病变中的应用[J]. 影像与介入, 2013, 20(36):108–109.Wang GS, Li ZH, Yang SK. The application of ultrasound speckle reduction imaging combined with tissue harmonic imaging for breast lesion[J]. China Modern Medicine, 2013, 20(36):108–109.
[15]Tan R, Xiao Y, Tang Q, et al. The Diagnostic Value of Micropure Imaging in Breast Suspicious Microcalcificaion[J]. Acad Radiol,2015, 22(11):1338–1343. doi: 10.1016/j.acra.2015.07.012.
[16]Machado P, Eisenbrey JR, Cavanaugh B, et al. New image processing technique for evaluating breast microcalcifications: a comparative study[J]. J Ultrasound Med, 2012, 31(6):885–893.
[17]Hellgren R, Dickman P, Lei fl and K, et al. Comparison of handheld ultrasound and automated breast ultrasound in women recalled after mammography screening[J]. Acta Radiol, 2017, 58(5):515–520.doi: 10.1177/0284185116665421.
[18]Chen L, Chen Y, Diao XH, et al. Comparative study of automated breast 3-D ultrasound and handheld B-mode ultrasound for differentiation of benign and malignant breast masses[J].Ultrasound Med Biol, 2013, 39(10):1735–1742. doi: 10.1016/j.ultrasmedbio.2013.04.003.
[19]朱罗茜, 包凌云, 朱庆庆, 等. 自动乳腺全容积成像技术及常规超声检查鉴别诊断乳腺影像报告和数据系统4类病灶良恶性的价值比较[J]. 中华医学超声杂志:电子版, 2016, 13(12):931–935. doi:10.3877/cma.j.issn.1672–6448.2016.12.011.Zhu LX, Bao LY, Zhu QQ, et al. Comparative study between automated breast volume scanner and conventional ultrasound in differentiation of benign and malignant breast imaging reporting and data system 4 breast lesions[J]. Chinese Journal of Medical Ultrasound:Electronic Edition, 2016, 13(12):931–935. doi: 10.3877/cma.j.issn.1672–6448.2016.12.011.
[20]Chae EY, Kim HH, Cha JH, et al. Evaluation of screening wholebreast sonography as a supplemental tool in conjunction with mammography in women with dense breasts[J]. J Ultrasound Med,2013, 32(9):1573–1578. doi: 10.7863/ultra.32.9.1573.
[21]Wang X, Huo L, He Y, et al. Early prediction of pathological outcomes to neoadjuvant chemotherapy in breast cancer patients using automated breast ultrasound[J]. Chin J Cancer Res, 2016,28(5):478–485.
[22]Shiina T, Nightingale K, Palmeri M, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: Basic principles and terminology[J]. Ultrasound Med Biol ,2015,41(5):1126–1147. doi: 10.1016/j.ultrasmedbio.2015.03.009.
[23]李俊来, 黄炎, 王知力, 等. 乳腺实时剪切波弹性成像的组织定征研究[J]. 中华医学超声杂志:电子版, 2011, 8(4):812–819. doi:10.3877/cma.j.issn.1672–6448.2011.04.029.Li JL, Huang Y, Wang ZL, et al. Real-time shear wave elastography in tissue characterization of breast[J]. Chinese Journal of Medical Ultrasound:Electronic Edition, 2011, 8(4):812–819. doi:10.3877/cma.j.issn.1672–6448.2011.04.029.
[24]Barr RG, Nakashima K, Amy D, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 2: breast[J]. Ultrasound Med Biol, 2015, 41(5):1148–1160. doi:10.1016/j.ultrasmedbio.2015.03.008.
[25]倪梁红, 张新书, 彭梅. 超声弹性成像鉴别乳腺小肿块良恶性的诊断价值[J]. 安徽医科大学学报, 2016, 51(2):277–279.Ni LH, Zhang XS, Peng M. Diagnostic value of ultrasound elasticity imaging in identi fi ng benign and malignant small breast masses[J].Acta Universitatis Medicinalis Anhui, 2016, 51(2):277–279.
[26]赵巧玲, 李芬, 盛薇, 等. 弹性应变率比值对乳腺肿块良恶性的诊断价值[J]. 中国普通外科杂志, 2011, 20(5):481–484.Zhao QL, Li F, Sheng W, et al. Diagnostic value of strain ratio measurement in breast neoplasm[J]. Chinese Journal of General Surgery, 2011, 20(5):481–484.
[27]Lee BE, Chung J, Cha ES, et al. Role of shear-wave elastography(SWE) in complex cystic and solid breast lesions in comparison with conventional ultrasound[J]. Eur J Radiol, 2015, 84(7):1236–1241. doi: 10.1016/j.ejrad.2015.04.005.
[28]周建桥, 周春, 詹维伟, 等. 超声弹性成像诊断乳腺良恶性病变的价值[J]. 诊断学理论与实践, 2011, 10(3):243–245.Zhou JQ, Zhou C, Zhan WW, et al. Value of sonoelastography in diagnosis of benign and malignant breast lesions[J]. Journal of Diagnostics Concepts & Practice, 2011, 10(3):243–245.
[29]Athanasiou A, Tardivon A, Tanter M, et al. Breast lesions:quantitative elastography with supersonic shear imaging preliminary results[J]. Radiology, 2010, 256(1):297–303. doi: 10.1148/radiol.10090385.
[30]Barr RG, Destounis S, Lackey LB 2nd, et al. Evaluation of breast lesions using sonographic elasticity imaging[J]. J Ultrasound Med,2012, 31(2):281–287.
[31]Hayashi M, Yamamoto Y, Sueta A, et al. Associations between elastography findings and clinicopathological factors in breast cancer[J]. Medicine(Baltimore), 2015, 94(50):e2290. doi: 10.1097/MD.0000000000002290.
[32]Hao Y, Guo X, Ma B, et al. Relationship between ultrasound elastography and myofibroblast distribution in breast cancer and its clinical significance[J]. Sci Rep, 2016, 6:19584. doi: 10.1038/srep19584.
[33]Greis C. Ultrasound contrast agents as markers of vascularity and microcirculation[J]. Clin Hemorheol Microcirc, 2009, 43(1/2):1–9.doi: 10.3233/CH-2009–1216.
[34]常婉英, 贾宛儒, 史俊妮, 等. 超声造影边缘特征对乳腺病灶诊断临床价值研究[J]. 中国超声医学杂志, 2016, 32(9):787–789.Chang WY, JIa WR, Shi JN, et al. Differential Diagnosis between Benign and Malignant Breast Lesions based on the Rim-Characteristics on Dynamic Contrast-enhanced Uitrasonography[J].Chinese Journal of Ultrasound in Medicine, 2016, 32(9):787–789.
[35]Zhao YX, Liu S, Hu YB, et al. Diagnostic and prognostic values of contrast-enhanced ultrasound in breast cancer: a retrospective study[J]. Onco Targets Ther, 2017, 10:1123–1129. doi: 10.2147/OTT.S124134.
[36]王小燕, 贺琰, 凌冰, 等. 乳腺肿块的超声造影特征与病理对照分析[J]. 中华肿瘤防治杂志, 2016, 23(10):667–671.Wang XY, He Y, Ling B, et al. Characteristic of contrast-enhanced ultrasound and its correlation with pathology for the breast tumor[J]. Chinese Journal of Cancer Prevention and Treatment,2016, 23(10):667–671.
[37]Luo J, Chen JD, Chen Q. Contrast-enhanced ultrasound improved performance of breast imaging reporting and data system evaluation of critical breast lesions[J]. World J Radiol, 2016, 8(6):610–617.doi: 10.4329/wjr.v8.i6.610.
[38]杨勇, 吕秀花, 崔光彬, 等. 超声造影与增强磁共振成像在乳腺良恶性肿瘤鉴别诊断中的应用[J]. 中国超声医学杂志, 2015,31(7):583–586.Yang Y, Lu XH, Cui GB, et al. Application of Contrast Enhanced Ultrasound and Dynamic Contrast Enhanced MRI in Differential Diagnosis of Benign and Malignant Breast Tumors[J]. Chinese Journal of Ultrasound in Medicine, 2015, 31(7):583–586.
[39]Kazi M, Suhani, Parshad R, et al. Fine-needle on retrospective review Aspiration Cytology (FNAC) in Breast Cancer: A Reappraisal Based of 698 Cases[J]. World J Surg, 2017,41(6):1528–1533. doi: 10.1007/s00268–017–3906-x.
[40]叶倩, 饶金, 易艳平, 等. 乳腺肿块细针穿刺细胞学与组织病理学对照分析[J]. 临床与实验病理学杂志, 2014, 30(1):19–21.doi:10.13315/j.cnki.cjcep.2014.01.005.Ye Q, Rao J, Yi YP, et al. Comparative analysis of fine needle aspiration cytology and histopathology in the diagnosis of breast masses[J]. Chinese Journal of Clinical and Experimental Pathology,2014, 30(1):19–21. doi:10.13315/j.cnki.cjcep.2014.01.005.
[41]Alkuwari E, Auger M. Accuracy of fi ne-needle aspiration cytology of axillary lymph nodes in breast cancer patients: a study of 115 cases with cytologic-histologic correlation[J]. Cancer, 2008,114(2):89–93. doi: 10.1002/cncr.23344.
[42]Barco I, Chabrera C, García-Fernández A, et al. Role of axillary ultrasound, magnetic resonance imaging, and ultrasound-guided fine-needleaspiration biopsy in the preoperative triage of breast cancer patients[J]. Clin Transl Oncol, 2017, 19(6):704–710. doi:10.1007/s12094–016–1589–7.