良性胆管狭窄(benign biliary stricture,BBS)是指非肿瘤因素引起的胆管(尤其是肝外胆管)管腔纤维组织增生、瘢痕挛缩。良性胆管狭窄是胆道外科的常见疾病,形成原因多由医源性胆管损伤、缺血、炎症、放化疗引起,处理棘手,患者并发症发生率高[1]。胆管损伤后,即使经满意修复,后期仍易出现狭窄。胆管损伤后狭窄主要表现为局部纤维化瘢痕形成,在这个过程中胆管上皮细胞的病理改变及免疫炎症反应失控发挥了重要作用,它们与释放的炎症因子相互作用的直接后果是大量炎症细胞浸润,导致成纤维细胞增殖旺盛,胶原过度合成,管腔瘢痕狭窄。Toll样受体4(TLR4)目前认为是启动天然免疫及炎症反应重要的细胞受体,在炎症中发挥重要作用。为了阐明TLR4在胆管狭窄形成中的作用,本研究检测了人正常胆管壁及狭窄胆管壁中的表达情况;通过建立TLR4基因缺陷小鼠(TLR4-/-)和野生型(TLR4+/+)小鼠肝外胆管缺血损伤修复模型,采用免疫组化方法对TLR4在愈合过程中的表达进行观察,探讨其在术后胆管狭窄形成过程中的作用及意义。
1 材料与方法
1.1 实验材料
收集了甘肃省人民医院普外一科2013年10月—2015年1月期间胆道良性狭窄患者12例,均为胆肠吻合口瘢痕,病程3个月至2年,均有黄疸病史,经影像学或手术证实存在胆总管或肝总管狭窄,在手术中取足量狭窄部位全层胆管壁组织。形成原因均为胆囊切除术(经腹腔镜10例,经开腹2例)胆管损伤后行Roux-en-Y式胆肠吻合术。4例胰十二指肠切除术患者的正常胆总管组织作为正常组。TLR4兔抗人多克隆抗体和TLR4兔抗鼠多克隆抗体购自甘肃宝信生物科技有限公司,免疫组织化学SP试剂盒购自甘肃宝信生物科技有限公司。
SPF级TLR4-/-和TLR4+/+雄性小鼠,5~6周,体质量25~30 g。购自美国匹兹堡大学。
1.2 实验方法
1.2.1 免疫组化方法 标本获取后,立即用等渗盐水冲洗表面的血液和胆汁,用10%甲醛固定,常规石蜡包埋,5 µm厚切片,常规脱蜡至脱水,高温修复后用动物血清封闭备用。加入兔抗人多克隆抗体和兔抗鼠多克隆抗体(TLR4为1:100),4 ℃过夜;次日以PBS溶液清洗后加入二抗(生物素羊抗兔多克隆抗体,浓度为1:300)及链霉菌生物素-过氧化物酶,室温下孵育30 min;最后以DAB显色、苏木精复染后封片。以0.01 mol/L PBS溶液代替一抗作为阴性对照。细胞膜或胞质内出现黄色或者褐色颗粒即为阳性细胞。显微镜(400倍)下随机计数10个视野的阳性细胞数和总细胞数,计算阳性细胞比例,并换算成阳性表达强度后再进行评价。阳性表达强度标准:“-”(<25%)、“+”(25%~50%)、“++”(51%~75%)及“+++”(>75%),其中“-”和“+”定义为表达阴性,“++”和“+++”定义为表达阳性。
1.2.2 动物实验方法 将实验大鼠按随机数字表法分为TLR4-/-模型组、假手术组、TLR4+/+模型组、TLR4-/-TLR4+/+假手术组,每组5只。术前各组体质量、精神状态、进食量差异无统计学意义(均P>0.05)。实验小鼠术前12 h禁食,6 h禁水。模型组1%戊巴比妥钠(30 mg/kg)腹腔注射麻醉后备皮,固定于清洁手术台上,腹部碘伏消毒,无菌条件下取上腹部正中切口打开腹腔,沿胃找十二指肠系膜缘,距十二指肠上缘约1.5cm处寻找到一条透明或半透明的条带状结构即为胆管。向上解剖一段长约0.8cm游离的胆总管,用2枚显微血管夹钳夹胆总管,术中间断注入生理盐水。3-0号丝线间断缝合关闭腹腔。术后不用抗生素,夹闭时间为48 h。术中或术后死亡的大鼠均重新补建模型,其中TLR4-/-模型组和TLR4+/+模型组小鼠在术中各死亡1只,均因在分离胆总管过程中不慎损伤门静脉出血导致死亡。术后观察指标动物的一般情况,包括进食、活动、反应状况、伤口情况。假手术组仅显露肝外胆管和游离胆总管,不做胆总管夹闭,余同模型组。术后48 h,模型组胆管及肝脏大体与光镜所见有明显改变,血清谷丙转氨酶(ALT)及胆红素水平显著升高,与假手术组相比有明显差异,提示用钳夹法建立小鼠胆管狭窄动物模型成功[2-3]。
1.3 统计学处理
采用SPSS 19.0软件进行统计学分析。统计数据以均数±标准差![]() 表示。对照组和病例组TLR4阳性细胞百分率采用t检验,同时两组之间的阳性率分别进行χ2检验,P<0.05为差异有统计学意义。
表示。对照组和病例组TLR4阳性细胞百分率采用t检验,同时两组之间的阳性率分别进行χ2检验,P<0.05为差异有统计学意义。
2 结 果
2.1 患者组织中TLR4的表达情况
免疫组织化学染色结果显示,正常组组织中TLR4罕有阳性染色;狭窄组中见大量阳性染色细胞,主要为胆管上皮细胞及浸润的淋巴细胞,中性粒细胞等炎症细胞,TLR4表达主要集中在细胞膜和细胞质中,染色呈棕黄色(图1)。狭窄组组织中TLR4的表达阳性率为83.33%(10/12),高于正常组的25.00%(1/4),差异有统计学意义(χ2=36.65,P<0.01)。
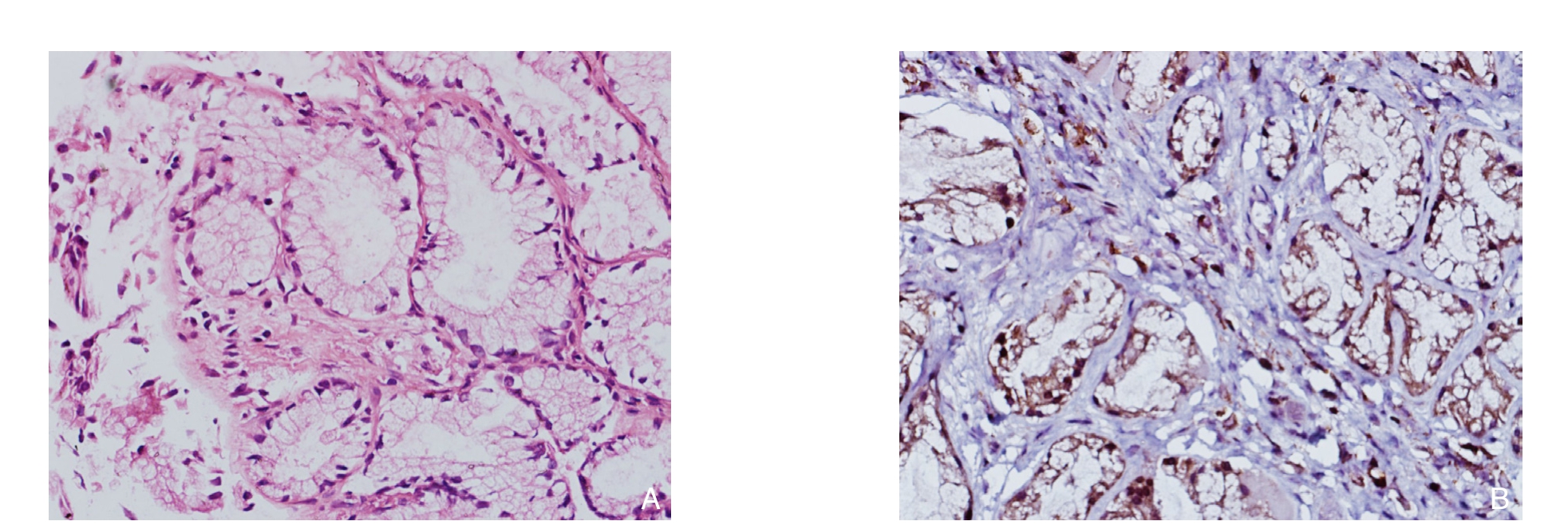
图1 免疫组化检测TLR4的表达(×400) A:正常胆管组织;B:狭窄胆管组织
Figure 1 Immunohistochemical staining for TLR4 expression (×400) A: Normal bile duct tissue; B: Bile duct tissue with biliary stricture
2.2 小鼠组织中胆管病理形态的变化
TLR4-/-模型组和TLR4+/+模型组小鼠结扎48 h后钳夹部位以上胆管明显扩张,肝脏、小肠淤胆严重。病理示TLR4+/+模型组小鼠缺血胆管管腔变小或闭塞,胆管黏膜坏死脱落,管壁纤维化增厚。肝脏可见少量肝细胞坏死,炎性细胞浸润;而TLR4-/-模型组胆管黏膜坏死及炎性细胞浸润均较TLR4+/+模型组轻(图2)。
2.3 小鼠肝功能变化情况
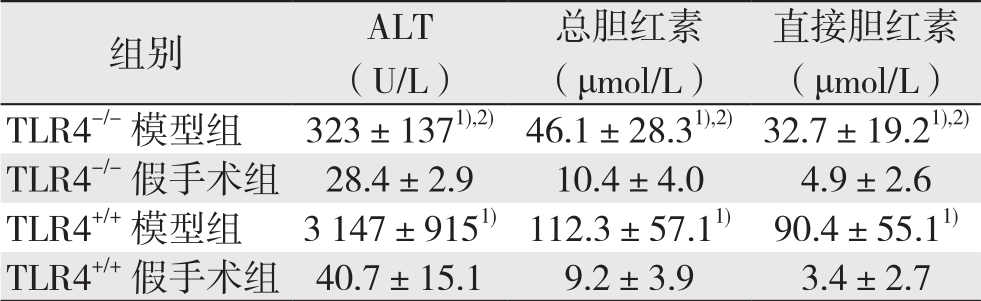
与各自的对照组比较,TLR4-/-与TL R4+/+模型组小鼠血清ALT水平明显升高(P<0.05),但TLR4-/-模型组低于TLR4+/+模型组(均P<0.05);TLR4-/-模型组和TLR4+/+模型组小鼠血清总胆红素、直接胆红素水平均明显升高于,TLR4-/-模型组(均P<0.05),但TLR4-/-模型组两者水平均低于TLR4+/+模型组(均P<0.05)。TLR4-/-和TLR4+/+假手术组血清ALT、总胆红素、直接胆红素水平无统计学差异(均P>0.05)(表1)。
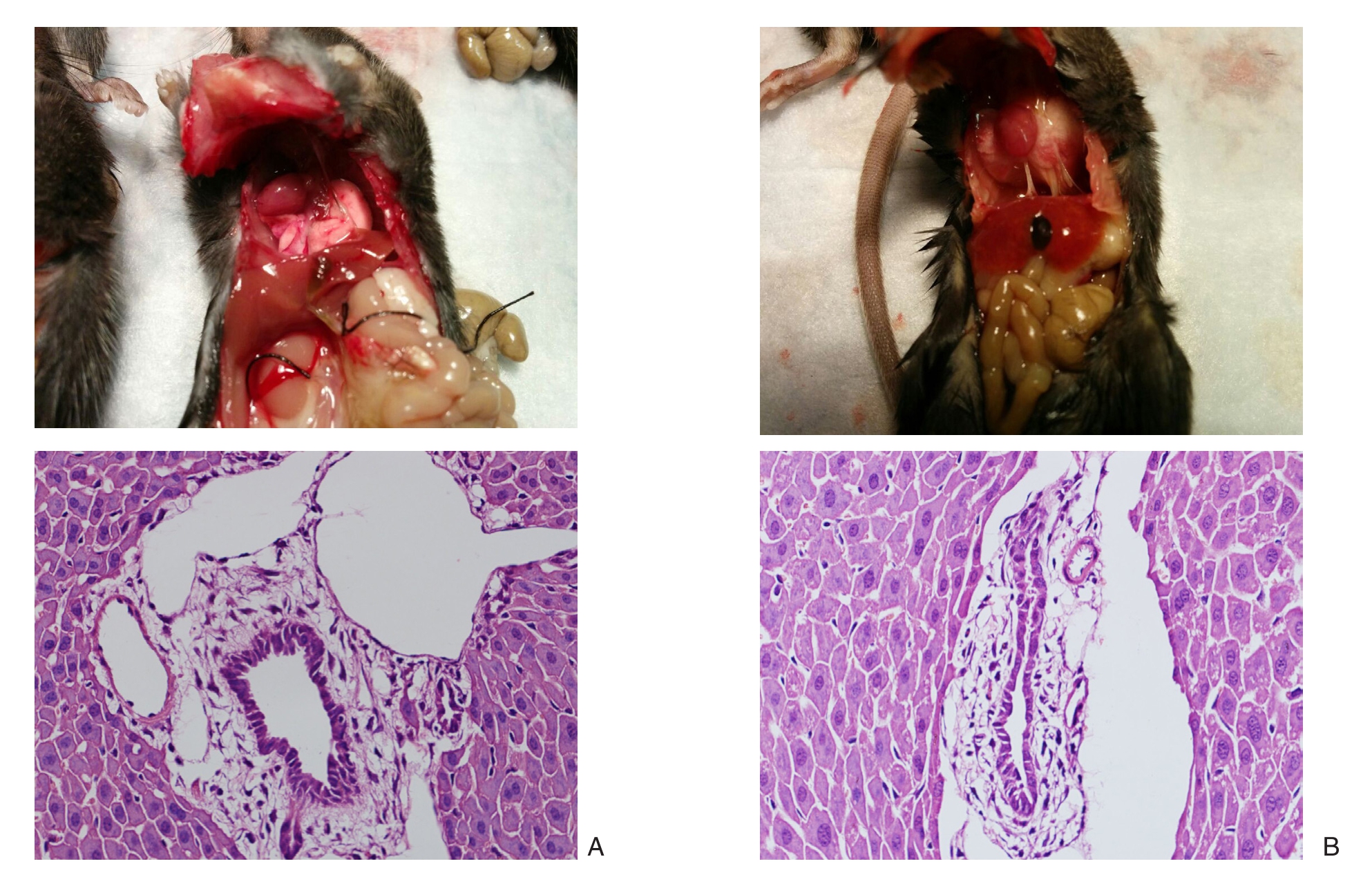
图2 大体与组织病理学观察(×400) A:TLR4-/-模型组;B:TLR4+/+模型组
Figure 2 Gross pathology and histopathological observations (×400) A: TLR4–/– model group; B: TLR4+/+ model group
表1 各组小鼠肝功能指标变化( ±s)
±s)
table 1 Changes in liver function parameters of each group of mice ( ±s)
±s)
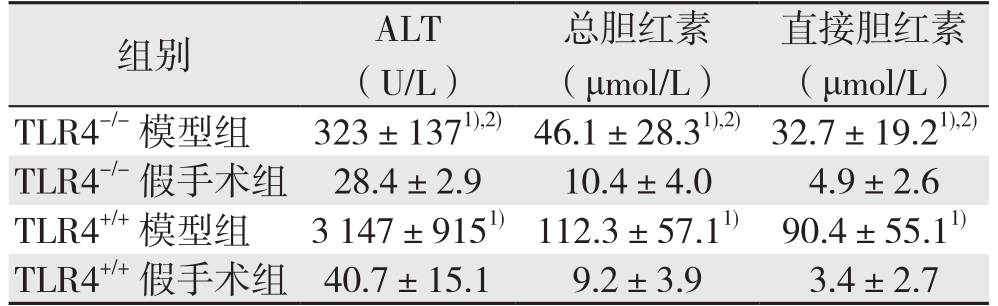
注:1)与各自的对照组比较,P<0.05;2)与TLR4+/+模型组比较,P<0.05
Note: 1) P<0.05 vs.corresponding control group; 2) P<0.05 vs.TLR4+/+ model group
3 讨 论
近年来,随着微创技术在胆道外科的广泛应用,经腹腔镜造成的医源性胆管损伤仍是肝胆外科难以避免的技术难题,目前该类损伤的发病率仍保持在0.5%~1%[4]。虽然手术重新建立了通道,但却不能解决瘢痕形成,再次狭窄的问题。因此对良性狭窄形成机制的研究具有重要意义。
胆管上皮细胞(biliary epithelial cell,BEC)是衬复在胆管内的上皮细胞,它构成胆道系统对病原微生物的第一道防线。BEC可以表达多种病原体识别受体,并激活细胞内的信号转导通路,启动内在的抗病原微生物的防御系统。BEC在病理条件下产生和分泌TNF-α[5-6]、IL-4、IL-6[7-8]、结缔组织生长因子等[9-10]。近年来,细胞因子和信号通路在胆道损伤修复中的作用受到了国内外学者的广泛关注,但是其具备的早期速发和链式加重的特点并不能很好的解释。胆管瘢痕狭窄是BEC对多种慢性损伤的修复反应,被认为是多种类型细胞,氧化应激、细胞因子和生长因子等一系列复杂作用的结果[11-12]。由于炎症反应及其炎症因子的作用在机体应激反应中具备双向性的特点,因此迫切需要深入了解炎症因子的始发点,找到控制其链式反应的“闸门”。
既往对胆道损伤狭窄的研究总是聚焦于细胞形态学或终末反应阶段的细胞因子,没有取得实质性进展。Toll样受体(Toll-like receptors,TLRs)的发现为这个问题的解决创造了可能。TLRs是一类可以识别病原体并迅速启动天然免疫反应的跨膜蛋白[13-15]。TLRs不仅识别外源性病原微生物,也可能被内源性配体激活,许多内源性配体与组织损伤有关,称为“损伤相关分子模式(damage-associated molecular patterns,DAMP)”,TLRs能够识别损伤与感染信号,因此它是连接组织损伤,感染和炎症的桥梁[16-18]。TLRs信号通路参与诱导BEC细胞因子和趋化因子的产生并介导BEC的增殖[19-21],而且BEC上调TLRs在原发性胆汁性肝硬化,原发性硬化性胆管炎,肝内外胆管结石的患者中被证实[22-23]。本实验也表明瘢痕狭窄的人胆管上皮细胞高表达TLR4,通过建立TLR4基因缺陷的小鼠缺血狭窄模型也发现,TLR4基因缺陷的小鼠在结扎48 h后表现出更轻的胆管炎症反应及肝功能的损伤。因此笔者推测肠道内毒素与其受体TLR4结合[24-26],激活下游信号转导路径,在机体内产生一系列的免疫应答,从而促成了胆管纤维化和狭窄的发生。但TLRs信号,免疫炎症反应,胆管纤维化之间的分子联系和调控机制仍需进一步的研究。
参考文献
[1]Geng ZM,Yao YM,Liu QG,et al.Mechanism of benign biliary stricture: a morphological and immunohistochemical study[J].World J Gastroenterol,2005,11(2):293–295.
[2]Zhao DF,Chen DZ,Lv JS,et al.Establishment of an animal model of biliary ischemic stenosis with clamping in mice[J].Transplant Proc,2008,40(5):1303–1305.doi: 10.1016/j.transproceed.2008.01.079.
[3]吕俊生,陈大志,秦建民,等.钳夹法建立小鼠胆管缺血狭窄模型[J].中华肝胆外科杂志,2006,12(6):419–421.doi:10.3760/cma.j.issn.1007–8118.2006.06.019.Lu JS,Chen DZ,Qin JM,et al.Establishment of an animal model of biliary ischemic stenosis with clamping in mice[J].Chinese Journal of Hepatobiliary Surgery 2006,12(6):419–421.doi:10.3760/cma.j.issn.1007–8118.2006.06.019.
[4]Mercado MA,Domínguez I.Classification and management of bile duct injuries[J].World J Gastrointest Surg,2011,3(4):43–48.doi:10.4240/wjgs.v3.i4.43.
[5]Yan C,Wang YH,Yu Q,et al.Clonorchis sinensis excretory/secretory products promote the secretion of TNF-alpha in the mouse intrahepatic biliary epithelial cells via Toll-like receptor[J].Parasit Vectors,2015,8:559.doi: 10.1186/s13071–015–1171–0.
[6]Akira S,Takeda K.Toll-like receptor signaling[J].Nat Rev Immunol,2004,4(7):499–511.
[7]陈莉萍,李晓武,郭毅斌,等.大鼠移植肝内胆管上皮细胞转化生长因子-β表达的变化[J].中华实验外科杂志,2007,24(1):47–48.doi:10.3760/j.issn:1001–9030.2007.01.018.Chen LP,Li XW,Guo YB,et al.Change of transforming growth factor-β expression in the biliary epithelial cells of rat liver graft[J].Chinese Journal of Experimental Surgery,2007,24(1):47–48.doi:10.3760/j.issn:1001–9030.2007.01.018.
[8]Demetris AJ,Lunz JG 3rd,Specht S ,et al.Biliary wound healing ,ductular reactions,and IL-6/ gp130 signaling in the development of liver disease[J].World J Gastroenterol,2006,12(22):3512–3522.
[9]Chen XM,O'Hara SP,Nelson JB,et al.Multiple TLRs are expressed in human cholangiocytes and mediate host epithelial defense responses to Cryptosporidium parvum via activation of NF-kappaB[J].J Immunol,2005,175(11):7447–7456.
[10]Nakanuma Y,Harada K,Sato Y,et al.Recent progress in the etiopathogenesis of pediatric biliary disease,particularly Caroli's disease with congenital hepatic fibrosis and biliary atresia[J].Histol Histopathol,2010,25(2):223–235.
[11]Fava G,Glaser S,Francis H,et al.The immunophysiology of biliary epithelium [J].Semin Liver Dis,2005,25(3):251–264.
[12]Chuang YH,Lan RY,Gershwin ME.The immunopathology of human biliary cell epithelium[J].Semin Immunopathol,2009,31(3):323–331.doi: 10.1007/s00281–009–0172–5.
[13]Varga MG,Peek RM.DNA Transfer and Toll-like Receptor Modulation by Helicobacter pylori[J].Curr Top Microbiol Immunol,2017,400:169–193.doi: 10.1007/978–3–319–50520–6_8.
[14]O'Hara JR,Feener TD,Fischer CD,et al.Campylobacter jejuni disrupts protective Toll-like receptor 9 signaling in colonic epithelial cells and increases the severity of dextran sulfate sodiuminduced colitis in mice[J].Infect Immun,2012,80(4):1563–1571.doi: 10.1128/IAI.06066–11.
[15]Satoh T,Akira S.Toll-Like Receptor Signaling and Its Inducible Proteins[J].Microbiol Spectr,2016,4(6).doi: 10.1128/microbiolspec.MCHD–0040–2016.
[16]Piccinini AM,Midwood KS.DAMPening inflammation by modulating TLR signalling[J].Mediators Inflamm,2010,pii:672395.doi: 10.1155/2010/672395.
[17]Bianchi ME.DAMPs,PAMPs and alarmins: all we need to know about danger[J].J Leukoc Biol,2007,81(1):1–5.
[18]Liew FY,Xu D,Brint EK,et al.Negative regulation of toll-like receptor-mediated immune responses[J].Nat Rev Immunol,2005,5(6):446–458.
[19]章权,于宏,吴硕东.胆管上皮细胞免疫功能的研究进展[J].世界华人消化杂志,2015,23(6):925–931.doi: 10.11569/wcjd.v23.i6.925.Zhang Q,Yu H,Wu SD.Immune function of biliary epithelial cells[J].World Chinese Journal of Digestology,2015,23(6):925–931.doi: 10.11569/wcjd.v23.i6.925.
[20]Fabris L,Strazzabosco M,Crosby HA,et al.Characterization and isolation of ductular cells coexpressing neural cell adhesion molecule and Bcl-2 from primary cholangiopathies and ductal plate malformations[J].Am J Pathol,2000,156(5): 1599–1612.
[21]Shaykhiev R,Behr J,Bals R.Microbial patterns signaling via Tolllike receptors 2 and 5 contribute to epithelial repair,growth and survival[J].PLoS One,2008,3(1):e1393.doi: 10.1371/journal.pone.0001393.
[22]Wang AP,Migita K,Ito M,et al.Hepatic expression of toll-like receptor 4 in primary biliary cirrhosis[J].J Autoimmun,2005,25(1):85–91.
[23]Guo J,Friedman SL.Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis[J].Fibrogenesis Tissue Repair,2010,3:21.doi: 10.1186/1755–1536–3–21.
[24]Xu MX,Wang M,Yang WW.Gold-quercetin nanoparticles prevent metabolic endotoxemia-induced kidney injury by regulating TLR4/NF-κB signaling and Nrf2 pathway in high fat diet fed mice[J].Int J Nanomedicine,2017,12:327–345.doi: 10.2147/IJN.S116010.
[25]Strier A,Kravarusic D,Coran AG,et al.The Effect of elevated intra-abdominal pressure on TLR4 signaling in intestinal mucosa and on intestinal bacterial translocation in a rat[J].J Laparoendosc Adv Surg Tech A,2016.[Epub ahead of print]
[26]Carrillo-Esper R.Innate immunity,Toll receptor and sepsis[J].Cir Cir,2003,71(3):252–258.