肝内胆管癌是肝脏常见的恶性肿瘤之一,起源于肝内细小胆管至肝管汇合处的胆管上皮细胞[1-2],约占原发性肝癌的10%[3],在过去20年间该病发病率和病死率在全球范围均呈现上升趋势,其病死率在美国印第安和阿拉斯加(0.13%)和亚洲泰国人群(0.14%)中最高,在欧洲人群(0.08%)和非洲人群(0.07%)最低[4-5]。Snail作为上皮间质转化(epithelial mesenchymal transition,EMT)过程中重要转录因子,可抑制上皮细胞E-钙黏蛋白(E-cadherin)表达,诱导细胞发生EMT。EMT是指上皮细胞转化为间质表型细胞,细胞失去上皮细胞表型和极性,细胞之间的粘连逐渐疏松,转化为具有高侵袭和运动能力的间质表型细胞[6-7],EMT被认为是导致肿瘤局部浸润和远处转移较为常见和重要的原因[8-9]。
本实验研究旨在探讨Snail在肝内胆管癌的表达和临床意义,了解Snail与肝内胆管癌侵袭转移临床病理特征的关系,初步探讨Snail在肝内胆管癌的研究意义,为进一步在体外研究和动物实验研究提供临床数据。
1 材料与方法
1.1 临床资料
临床患者资料来自于昆明医科大学第二附属医院肝胆外科二病区1999年12月—2010年1月期间55例肝内胆管癌患者,均接受根治性手术切除术,取肝内胆管癌肿瘤组织以及邻近的癌旁正常胆管组织,全部患者签署知情同意书,并获昆明医科大学第二附属医院伦理委员会批准;55例患者均无全身远处转移,男28例,女27例;年龄≥70岁者15例,年龄<70岁者40例;血清CA19-9≥37 ng/mL者30例,CA19-9<37 ng/mL者25例;血清CEA≥5 ng/mL者40例,CEA<5 ng/mL者15例;肿瘤单发者42例,多发者13例。TNM分期标准采用国际抗癌联盟(UICC)与美国癌症联合委员会(AJCC)条例。病理组织评分标准采用国际卫生组织条例:肿瘤中低分化者31例,高分化者24例。根据术中所见及术后淋巴结病理检查,有淋巴结转移者29例,无淋巴结转移者26例。术后病检结果均为肝内胆管癌,全部患者术前未接受化疗和放疗。随访日期到2015年1月1日结束,失访6例,平均随访期为(22.6±17.3)(3~60)个月
1.2 免疫组化
临床病理组织采用4%多聚甲醛(上海翊圣生物科技有限公司)固定、石蜡包埋、制做组织切片,90 ℃烤片2 h,二甲苯溶液(上海翊圣生物)浸泡2次各15 min,依次不同浓度乙醇(100%、95%、90%、75%、70%)各5 min,抗原修复采用柠檬酸钠(上海翊圣生物,10 mmol/L,pH 6.0)100 ℃维持10 min,后室温冷却30 min,3%过氧化氢(南京凯基生物)作用20 min阻断内源性过氧化物酶,5%牛血清蛋白(上海翊圣生物)封闭2 h,孵育一抗兔抗Snail单克隆抗体(1:500,英国Abcam公司,货号ab180714),4 ℃过夜,孵育二抗(山羊抗兔二抗,上海翊圣生物科技有限公司)20 min,DAB显色(南京凯基生物),显微镜下控制,苏木素(南京凯基生物)细胞核复染,酒精水化及二甲苯透明,中性树胶(上海翊圣生物)封片,奥林巴斯光学(日本,DP71)显微镜下镜检采集数据。
1.3 免疫组化结果评分
评分方法参考复旦大学中山医院肝癌研究所出版文献标准[10-11],采用Image pro plus软件分析,综合染色面积和强度评分。染色面积标准:棕色面积>50%为1分,棕色<50%位0分。染色强度标准:棕黑色染色为2分,较浅棕色为1分,无染色为0分。结果分别给2名病理科医师观察,染色面积和强度评分相加,总分≥2分认为是高表达,低于2认为是低表达。
1.4 统计学处理
数据处理采用SPSS 21.0软件,计数资料采用χ2检验,癌和癌旁Snail表达比较采用配对t检验。绘制Kaplan-Meier总体生存曲线和累计复发曲线,Log-rank检验分析总体生存率和累计复发率差异,采用Cox回归模型进行多因素生存分析。检验结果取双侧,检验水准α=0.05,P<0.05为差异有统计学意义。
2 结 果
2.1 Snail在肝内胆管癌的表达及其与临床病理特征的关系
免疫组化法检测55例肝内胆管癌病理组织,结果表明在肿瘤组织中(图1A-B)表达明显高于癌旁组织(图1C-D);Snail染色主要位于细胞核,在细胞质和细胞膜中未见表达,经免疫组化评分分析肿瘤组织与癌旁组织结果分别为2.764±0.844、0.914+0.765,差异有统计学意义(t=12.575,P<0.001)(图1E);其中肿瘤组织高表达率为48.6%(27/55),癌旁组织高表达率为18.0%(10/55),两者间差异有统计学意义(χ2=11.770,P=0.001)(图1F)。
将Snail定量表达与肝内胆管癌病例病理特征相结合分析,结果表明过表达的Snail患者与低表达的Snail患者在淋巴结转移、TNM分期、肿瘤分化、微血管侵犯以及复发之间存在差异(表1)。
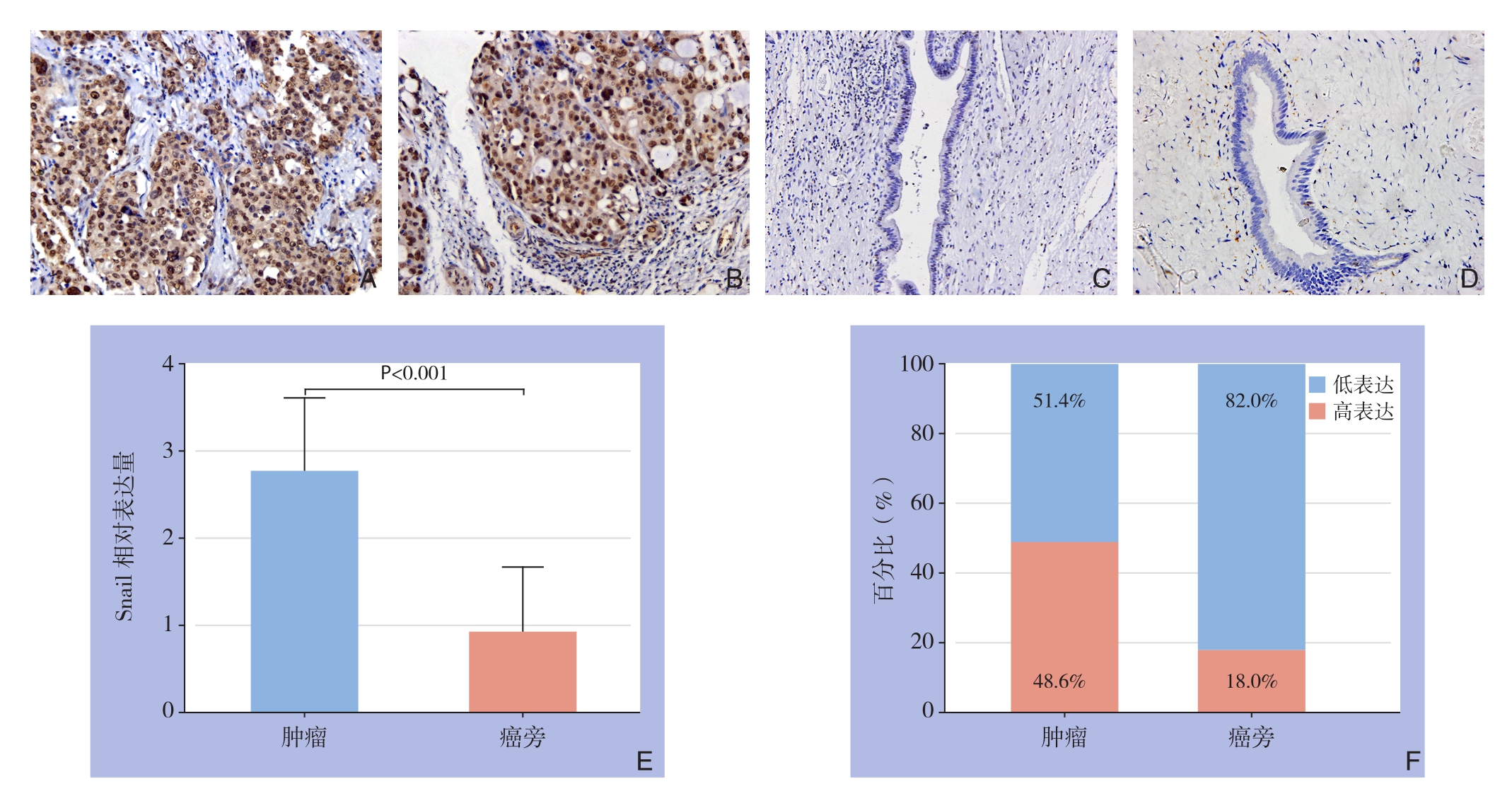
图1 Snail表达免疫组化检测结果 A-B:肿瘤组织(×200);C-D:癌旁组织(×200);E:Snail相对表达量比较;F:Snail高表达百分比比较
Figure 1 Results of immunohistochemical staining for Snail expressions A–B: Tumor tissue (×200); C–D: Peritumoral tissue (×200);E: Comparison of relative Snailexpression levels; F: Comparison of percentages of high Snail expression
2.2 Snail与55例肝内胆管癌病例生存分析
S n a i l免疫组化染色结果与生存分析结果表明,过表达Snail的病例术后总体生存率较低(P=0.018)(图2A),术后累计复发率较高(P=0.032)(图2B)。单因素总体生存率和累计复发率分析结果表明TNM分期、微血管侵犯淋巴结转移、以及Snail染色可预测肝内胆管癌患者预后(均P<0.05)(表2);多因素Cox回归模型分析结果表明微血管侵犯、淋巴结转移以及Snail 表达可是肝内胆管癌患者预后的独立影响因素(均P<0.05)(表3)。
表1 Snail表达与肝内胆管癌患者临床病理特征的关系分析[n(%)]
Table 1 Analysis results of relations of snail expression with cliniopathologic features ICC patients [n(%)]
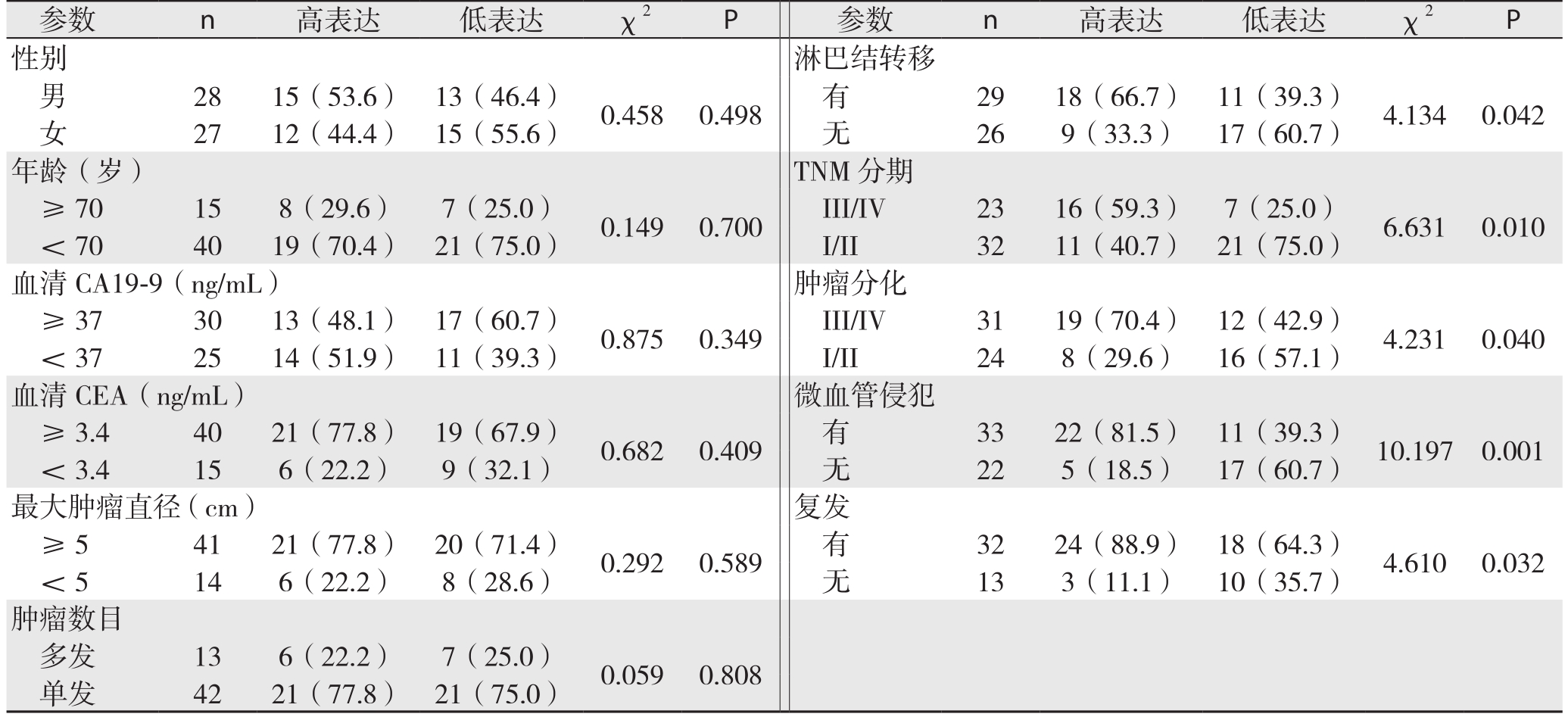
参数 n 高表达 低表达 χ2 P 参数 n 高表达 低表达 χ2 P性别 淋巴结转移男28 15(53.6) 13(46.4) 0.458 0.498 有 29 18(66.7) 11(39.3) 4.134 0.042女27 12(44.4) 15(55.6) 无 26 9(33.3) 17(60.7)年龄(岁) TNM分期≥70 15 8(29.6) 7(25.0) 0.149 0.700 III/IV 23 16(59.3) 7(25.0) 6.631 0.010<70 40 19(70.4) 21(75.0) I/II 32 11(40.7) 21(75.0)血清CA19-9(ng/mL) 肿瘤分化≥37 30 13(48.1) 17(60.7) 0.875 0.349 III/IV 31 19(70.4) 12(42.9) 4.231 0.040<37 25 14(51.9) 11(39.3) I/II 24 8(29.6) 16(57.1)血清CEA(ng/mL) 微血管侵犯≥3.4 40 21(77.8) 19(67.9) 0.682 0.409 有 33 22(81.5) 11(39.3) 10.197 0.001<3.4 15 6(22.2) 9(32.1) 无 22 5(18.5) 17(60.7)最大肿瘤直径(cm) 复发≥5 41 21(77.8) 20(71.4) 0.292 0.589 有 32 24(88.9) 18(64.3) 4.610 0.032<5 14 6(22.2) 8(28.6) 无 13 3(11.1) 10(35.7)肿瘤数目多发 13 6(22.2) 7(25.0) 0.059 0.808单发 42 21(77.8) 21(75.0)
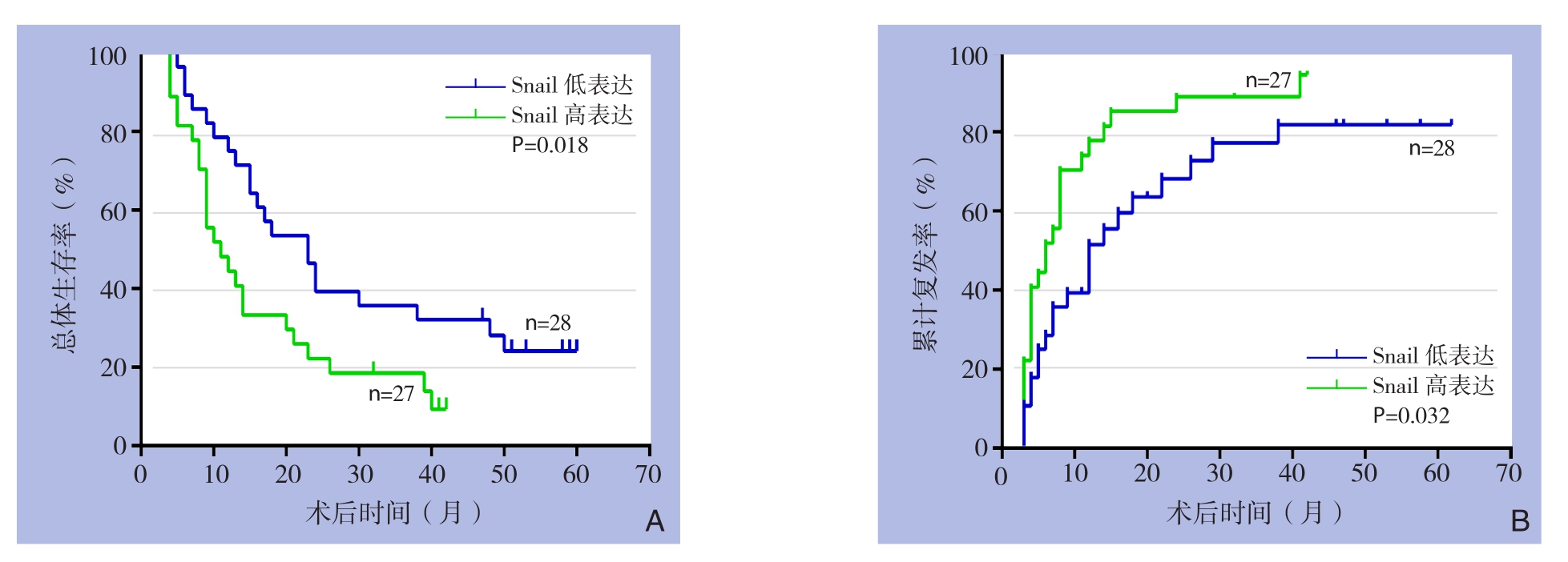
图2 Snail表达与患者生存的关系 A:总体生存率;B:累计复发率
Figure 2 Relation of Snail expression and survival of the patients A: Overall survival rate; B: Cumulative recurrence rate
表2 单因素总体生存率和累计复发率分析结果
Table 2 Univariate analysis of factors for overall survival and cumulative recurrence rates
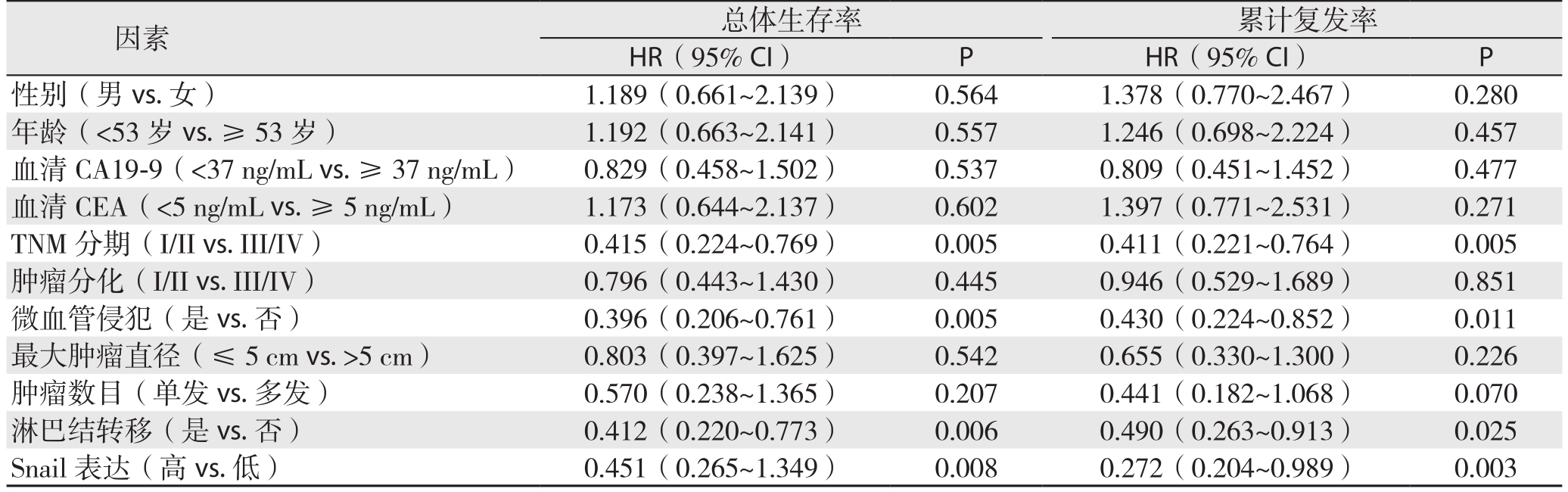
因素 总体生存率 累计复发率HR(95% CI) P HR(95% CI) P性别(男vs.女) 1.189(0.661~2.139) 0.564 1.378(0.770~2.467) 0.280年龄(<53岁vs.≥53岁) 1.192(0.663~2.141) 0.557 1.246(0.698~2.224) 0.457血清CA19-9(<37 ng/mL vs.≥37 ng/mL) 0.829(0.458~1.502) 0.537 0.809(0.451~1.452) 0.477血清CEA(<5 ng/mL vs.≥5 ng/mL) 1.173(0.644~2.137) 0.602 1.397(0.771~2.531) 0.271 TNM分期(I/II vs.III/IV) 0.415(0.224~0.769) 0.005 0.411(0.221~0.764) 0.005肿瘤分化(I/II vs.III/IV) 0.796(0.443~1.430) 0.445 0.946(0.529~1.689) 0.851微血管侵犯(是vs.否) 0.396(0.206~0.761) 0.005 0.430(0.224~0.852) 0.011最大肿瘤直径(≤5cm vs.>5cm) 0.803(0.397~1.625) 0.542 0.655(0.330~1.300) 0.226肿瘤数目(单发vs.多发) 0.570(0.238~1.365) 0.207 0.441(0.182~1.068) 0.070淋巴结转移(是vs.否) 0.412(0.220~0.773) 0.006 0.490(0.263~0.913) 0.025 Snail表达(高vs.低) 0.451(0.265~1.349) 0.008 0.272(0.204~0.989) 0.003
表3 多因素Cox回归模型分析结果
Table 3 Analysis results of multivariate Cox regression model
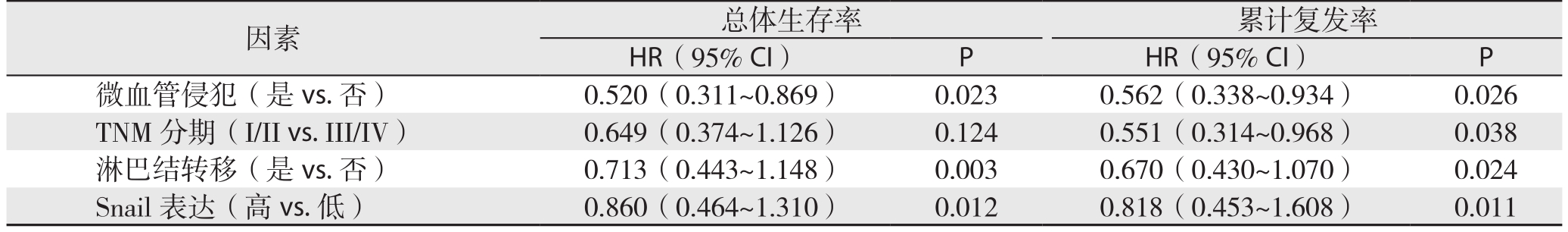
因素 总体生存率 累计复发率HR(95% CI) P HR(95% CI) P微血管侵犯(是vs.否) 0.520(0.311~0.869) 0.023 0.562(0.338~0.934) 0.026 TNM分期(I/II vs.III/IV) 0.649(0.374~1.126) 0.124 0.551(0.314~0.968) 0.038淋巴结转移(是vs.否) 0.713(0.443~1.148) 0.003 0.670(0.430~1.070) 0.024 Snail表达(高vs.低) 0.860(0.464~1.310) 0.012 0.818(0.453~1.608) 0.011
3 讨 论
肝内胆管癌作为一种侵袭性较强的恶性肿瘤,预后较差、有效治疗措施有限[12-13],高侵袭和转移是其导致死亡的最主要原因之一[12],选取合理有效的分子标记物评估肝内胆管癌患者预后与早期复发,对肝内胆管癌患者具有重要的临床治疗指导意义,大量研究[14-16]证实EMT可促进肿瘤细的胞侵袭和转移能力,而在EMT过程中Snail作为关键的转录因子[17],其可促进肿瘤EMT致使肿瘤细胞侵袭和转移能力大大提高,诱发肿瘤细胞远处转移[18-19]。
核转录调节因子S n a i l,能够结合到E-cadherin基因DNA中启动子区域[20]以及通过表观遗传学修饰[21]抑制E-cadherin的表达,诱导细胞EMT,在众多促进肿瘤侵袭的研究中表明,不同的分子通过不同的信号通路导致Snail因子异常增加,可加速肿瘤细胞EMT的发生,Ke等[22]在CD151分子对肝细胞肝癌的肿瘤侵袭转移研究中,发现CD151可通过PI3K/Akt/Snali信号通路促进肿瘤细胞EMT;此外在体外肝内胆管癌细胞研究中,采用RNA干扰技术沉默Snail蛋白表达后细胞侵袭转移能力减低[11];吴天山等[23]对胰腺癌患者行病理标本免疫组化染色,结合随访资料分析Snail与EMT指标密切相关,是胰腺癌转移相关的重要指标。本研究结果表明,Snail在肝内胆管癌病理组织中呈现过表达,临床病理特征分析结果表明过表达的Snail与侵袭转移病理特征相关,结合既往研究,笔者推测Snail在肝内胆管癌中过表达可能会诱导肿瘤细胞EMT改变,致使肿瘤细胞侵袭和转移能力增加,这为目前肿瘤的分子靶向提供了部分临床数据,当靶向沉默Snail后可能会降低细胞侵袭转移能力,缓解患者的远处转移和肿瘤复发,为患者带来新的治疗机会。
在Snail临床随访研究中,Kong等[24]对44例肝门部胆管癌进行了临床随访研究,结果表明Snail可预测较差的预后,作为预后指标;在103例胃癌病例的临床随访研究中,结果表明Snail能够作为一种合理的预后因子预测胃癌的进展,并可作为胃癌的干预靶点之一[25]。在此,我们的随访生存分析结果表明,过表达的Snail肝内胆管癌患者中,其术后总体生存率较低,且过表达Snail患者的术后累计复发率较高,在单因素分析和多因素Cox回归模型分析结果表明Snail染色可作为预测肝内胆管癌患者预后的指标,这与Snail在其他肿瘤中的研究结果类似,这对指导患者术后常规进行Snail免疫组化染色评估肝内胆管癌患者的预后具有重要的意义,为早期进行生物治疗提供有力的数据,同样为降低患者术后复发率和病死率都提供有力的支持。
综上所述,本研究实验结论为Snail在肝内胆管癌中表现为过表达,过表达的Snail与肝内胆管癌侵袭转移临床病理特征相关,虽Snail的具体作用机制需要我们更深层次的探索,但根据本研究结果推断,Snail有可能做为预测肝内胆管癌患者预后因子,并可望作为生物治疗的作用靶点。
参考文献
[1]Yang LX,Gao Q,Shi JY,et al.Mitogen-activated protein kinase kinase kinase 4 deficiency in intrahepatic cholangiocarcinoma leads to invasive growth and epithelial-mesenchymal transition[J].Hepatology,2015,62(6):1804–1816.doi: 10.1002/hep.28149.
[2]杭轶,杨小勇,李文美,等.肝内胆管癌与肝细胞癌临床特征的比较研究[J].中国普通外科杂志,2015,24(2):175–179.doi:10.3978/j.issn.1005–6947.2015.02.004.Hang Y,Yang XY,Li WM,et al.Comparative study of clinical features between intrahepatic cholangiocarcinoma and hepatocellular carcinoma[J].Chinese Journal of General Surgery,2015,24(2):175–179.doi:10.3978/j.issn.1005–6947.2015.02.004.
[3]Zhang GW,Lin JH,Qian JP,et al.Identification of risk and prognostic factors for patients with clonorchiasis-associated intrahepatic cholangiocarcinoma[J].Ann Surg Oncol,2014,21(11):3628–3637.doi: 10.1245/s10434–014–3710–x.
[4]Weber SM,Ribero D,O'Reilly EM,et al.Intrahepatic cholangiocarcinoma: expert consensus statement[J].HPB (Oxford),2015,17(8):669–680.doi: 10.1111/hpb.12441.
[5]Brown KM,Parmar AD,Geller DA.Intrahepatic cholangiocarcinoma[J].Surg Oncol Clin N Am,2014,23(2):231–246.doi: 10.1016/j.soc.2013.10.004.
[6]Zhou J,Wang J,Zhang N,et al.Identification of biomechanical force as a novel inducer of epithelial-mesenchymal transition features in mechanical stretched skin[J].Am J Transl Res,2015,7(11):2187–2198.
[7]Seton-Rogers S.Epithelial-mesenchymal transition: Untangling EMT's functions[J].Nat Rev Cancer,2016,16(1):1.doi: 10.1038/nrc.2015.6.
[8]Zhou S,Shen Y,Wang L,et al.Epithelial-mesenchymal transition and mesenchymal-epithelial transition response during differentiation of growth-plate chondrocytes in endochondral ossification[J].Int J Clin Exp Med,2015,8(8):12076–12085.
[9]Brivio S,Cadamuro M,Fabris L,et al.Epithelial-to-Mesenchymal Transition and Cancer Invasiveness: What Can We Learn from Cholangiocarcinoma?[J].J Clin Med,2015,4(12):2028–2041.doi:10.3390/jcm4121958.
[10]Ke AW,Zhang PF,Shen YH,et al.Generation and characterization of a tetraspanin CD151/integrin α6β1-binding domain competitively binding monoclonal antibody for inhibition of tumor progression in HCC[J].Oncotarget,2016,7(5):6314–6322.doi: 10.18632/oncotarget.6833.
[11]Huang XY,Zhang C,Cai JB,et al.Comprehensive multiple molecular profile of epithelial mesenchymal transition in intrahepatic cholangiocarcinoma patients[J].PLoS One,2014,9(5):e96860.doi: 10.1371/journal.pone.0096860.
[12]Padia SA.Intrahepatic Cholangiocarcinoma[J].Tech Vasc Interv Radiol,2015,18(4):227–235.doi: 10.1053/j.tvir.2015.07.006.
[13]Spolverato G,Yakoob MY,Kim Y,et al.Impact of complications on long-term survival after resection of intrahepatic cholangiocarcinoma[J].Cancer,2015,121(16):2730–2739.doi:10.1002/cncr.29419.
[14]Ma JL,Zeng S,Zhang Y,et al.Epithelial-mesenchymal transition plays a critical role in drug resistance of hepatocellular carcinoma cells to oxaliplatin[J].Tumour Biol,2016,37(5):6177–6184.doi:10.1007/s13277–015–4458–z.
[15]Guo Q,Ning F,Fang R,et al.Endogenous Nodal promotes melanoma undergoing epithelial-mesenchymal transition via Snail and Slug in vitro and in vivo[J].Am J Cancer Res,2015,5(6):2098–2112.
[16]Sung WJ,Park KS,Kwak SG,et al.Epithelial-mesenchymal transition in patients of pulmonary adenocarcinoma: correlation with cancer stem cell markers and prognosis[J].Int J Clin Exp Pathol,2015,8(8):8997–9009.
[17]Liu L,Dai Y,Chen J,et al.Maelstrom promotes hepatocellular carcinoma metastasis by inducing epithelial-mesenchymal transition by way of Akt/GSK-3beta/Snail signaling[J].Hepatology,2014,59(2):531–543.doi: 10.1002/hep.26677.
[18]Wang YL,Zhao XM,Shuai ZF,et al.Snail promotes epithelialmesenchymal transition and invasiveness in human ovarian cancer cells[J].Int J Clin Exp Med,2015.8(5):7388–7393.
[19]Giannelli G,Koudelkova P,Dituri F,et al.Role of epithelial to mesenchymal transition in hepatocellular carcinoma[J].J Hepatol,2016,65(4):798–808.doi: 10.1016/j.jhep.2016.05.007.
[20]Pilli VS,Gupta K,Kotha BP,et al.Snail-mediated Cripto-1 repression regulates the cell cycle and epithelial-mesenchymal transition-related gene expression[J].FEBS Lett,2015,589(11):1249–1256.doi: 10.1016/j.febslet.2015.04.005.
[21]Feng J,Cen J,Li J,et al.Histone deacetylase inhibitor valproic acid(VPA) promotes the epithelial mesenchymal transition of colorectal cancer cells via up regulation of Snail[J].Cell Adh Migr,2015,9(6):495–501.doi: 10.1080/19336918.2015.1112486.
[22]Ke AW,Shi GM,Zhou J,et al.CD151 amplifies signaling by integrin alpha6beta1 to PI3K and induces the epithelialmesenchymal transition in HCC cells[J].Gastroenterology,2011,140(5):1629–1641.e15.doi: 10.1053/j.gastro.2011.02.008.
[23]吴天山,郭飞.细胞核Snail因子在胰腺癌上皮-间质转化中的作用[J].中国普通外科杂志,2015,24(3):426–428.doi:10.3978/j.issn.1005–6947.2015.03.023.Wu TS,Guo F.Role of nucleus factor Snail on epithelialmesenchymal transformation in pancreatic cancer[J].Chinese Journal of General Surgery,2015,24(3):426–428.doi:10.3978/j.issn.1005–6947.2015.03.023.
[24]Kong D,Liang J,Li R,et al.Prognostic significance of snail expression in hilar cholangiocarcinoma[J].Braz J Med Biol Res,2012,45(7): 617–624.
[25]He H,Chen W,Wang X,et al.Snail is an independent prognostic predictor for progression and patient survival of gastric cancer[J].Cancer Sci,2012,103(7):1296–1303.doi: 10.1111/j.1349–7006.2012.02295.x.