大约有15%的乳腺癌患者ER、PR、HER-2均表达阴性,称作三阴性乳腺癌(triple negative breast cancer,TNBC)[1]。TNBC较其他类型乳腺癌预后差,病理类型多为浸润性导管癌,具有侵袭性强、易复发、易转移、生存期短和病死率高等特点[2-3]。TNBC治疗手段匮乏,化疗是其唯一有效综合治疗手段[4-6]。BCL11A基因,最早于2000年在T(2;14)(p16;q32.3)异位的慢性淋巴细胞白血病中发现[7]被发现,位于人类基因组的2p13位点,在物种间高度保守,表达于胎肝、骨髓、脑组织中,并在B淋巴细胞[8]、早期T祖细胞、淋巴系祖细胞以及造血干细胞等造血组织中高表达,在B淋巴细胞发育增殖中起到重要的作用[9-11]。Khaled等[12]为了识别TNBC潜在致癌基因,从欧美人群的肿瘤大数据库中筛选出并证实BCL11A基因是TNBC的致癌基因。本研究旨在研究BCL11A在我国TNBC中的表达水平及其与其他类型乳腺癌表达水平的差异,初步探讨新辅助化疗对BCL11A表达的影响,分析其在TNBC中与临床病理参数之间的相关性,为寻求TNBC靶向治疗提供参考。
1 资料与方法
1.1 标本来源
收集2013年1月—2015年12月收治在湘雅医院乳腺科可手术的浸润性乳腺癌患者的临床病理资料,从TNBC患者中随机抽取有新辅助化疗前后2次病理结果的43例TNBC,未经新辅助化疗的非TNBC中随机抽取49例乳腺管腔型乳腺癌,50例HER-2阳性型乳腺癌。
1.2 方法及试剂
应用免疫组织化学方法检测BCL11A蛋白。兔抗人BCL11A单克隆抗体(ab191402),购于英国Abcam公司,免疫组化检测试剂盒(sp9000,羊抗兔),购于北京中杉金桥生物有限公司,按照试剂盒说明书进行造作。
1.3 结果判断
BCL11A在细胞浆和细胞核中表达,染色呈现出黄色、棕黄色或棕褐色。定性分析:每张切片均随机选择5个高倍镜视野(400倍),以细胞核和细胞质呈清晰棕黄色着色作为阳性表达细胞,记录阳性细胞所占比例及染色强度。评分标准:按阳性细胞数比例计0、1、2、3、4分,分别对应无阳性细胞、≤25%、26%~50%、51%~75%、>75%。按阳性细胞着色程度记分为0、1、2、3分,依次对应无着色、淡黄色、棕黄色、棕褐色。上述两种记分相加,取2名病理科教授评分平均值。0~1分记为阴性(-);2~3分记为弱阳性(+);4~5分记为中等阳性(++);6~7分记为强阳性(+++)。定量分析:为了更客观的评价抗体的特异性,将上述染色的切片,在显微镜高倍镜视野(400倍)下定位后,用彩色摄像机提取细胞图像输入Image-Pro Plus(IPP)图像处理分析软件,通过图像分割、统计,获得各例病理切片细胞BCL11A的平均吸光光密度值(u值),u值越大,染色越深。
1.4 统计学处理
应用SPSS 19.0软件进行统计学分析。计数资料均采用χ2检验,所有计量数据先采用方差齐性分析是否属于正态分布,采用配对t检验分析新辅助化疗前后BCL11A表达水平的差异,采用单样本ANOVA方差分析对3类乳腺癌的BCL11A表达水平的差异进行定量分析。检验水准取α=0.05,P<0.05(双侧)为差异有统计学意义。
2 结 果
2.1 BCL11A在不同类型乳腺癌中的表达情况
对4 3例TNBC(化疗前)、49例管腔型、50例HER-2阳性乳腺癌组织BCL11A阳性表达率分别进行定性与定量分析(图1)(表1)。结果显示,BCL11A在TNBC组、管腔型组、HER-2阳性组的阳性表达率分别为76.7%(33/43)、26.5%(13/49)、32.0%(16/50),TNBC组BCL11A阳性表达率明显高于管腔型组和HER-2阳性组(均P<0.05);TNBC组BCL11A表达量u值明显高于管腔型组、HER-2阳性组(均P<0.05)(图2)。
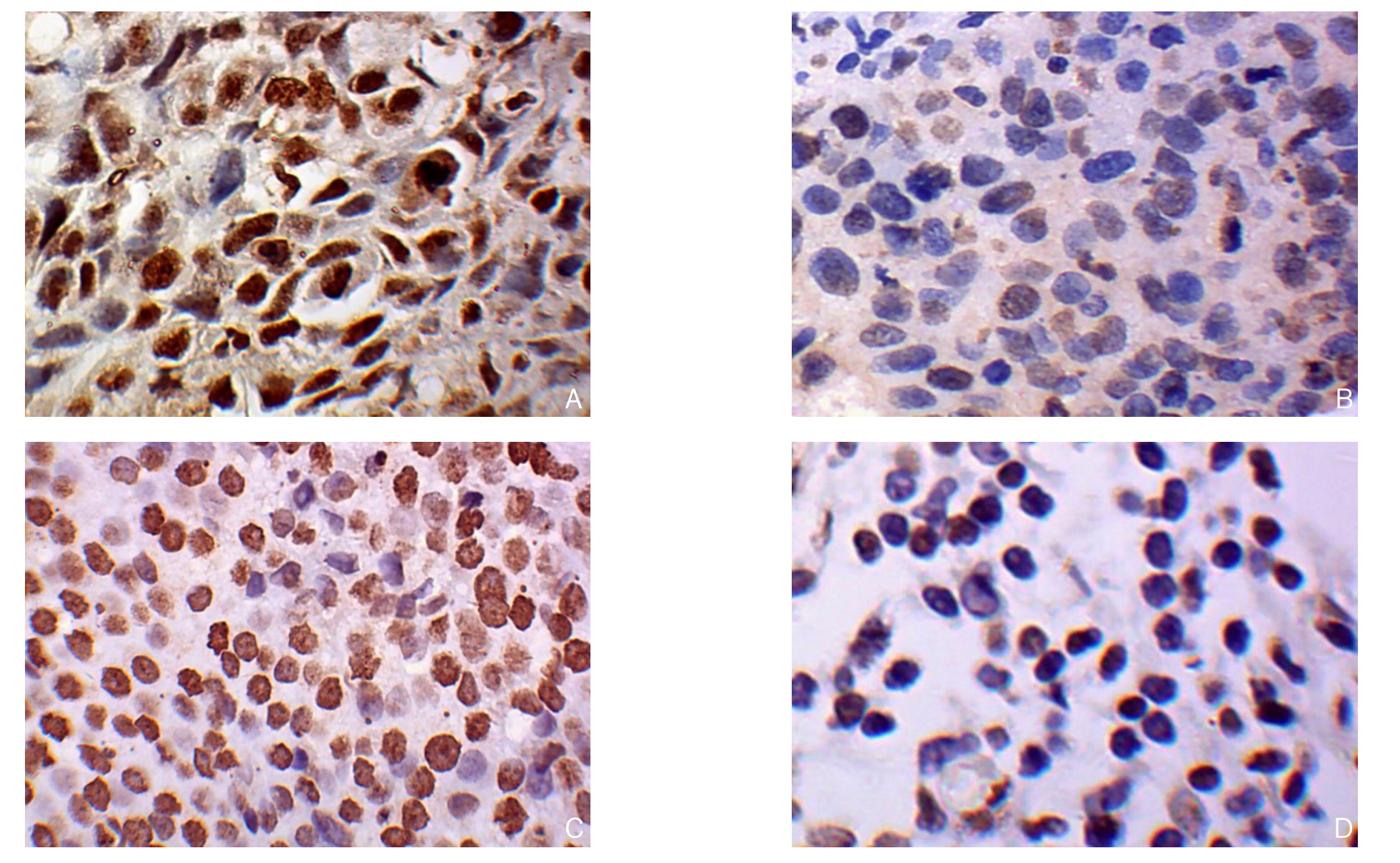
图1 BCL11A免疫组化染色(×400) A:BCL11A在TNBC中阳性表达;B:BCL11A在TNBC阴性表达;C:BCL11A在非TNBC中阳性表达;D:BCL11A在非TNBC中阴性表达
Figure 1 Immunohistochemical staining for BCL11A expression (×400) A: Positive BCL11A expression in TNBC tissue; B: Negative BCL11A expression in TNBC tissue; C: Positive BCL11A expression in non-TNBC tissue; D: Negative BCL11A expression in non-TNBC tissue
表1 BCL11A在不同类乳腺癌组织中的阳性表达率[n(%)]
Table 1 Positive expression rates of BCL11A in different types of breast cancer tissues [n (%)]
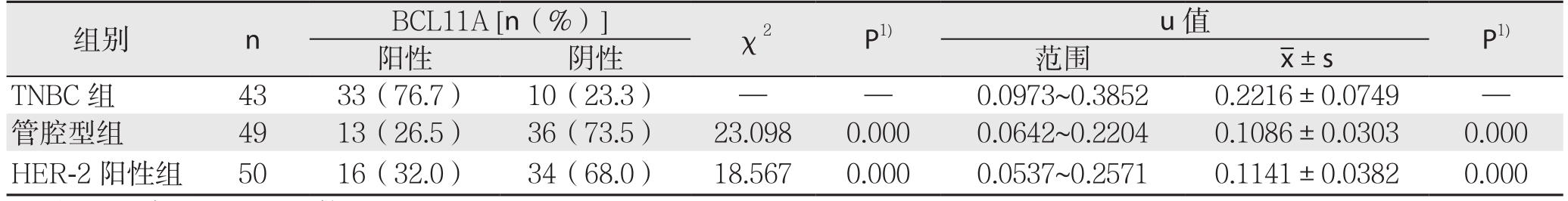
注:1)与TNBC组比较
Note: 1) Comparison with TNBC group
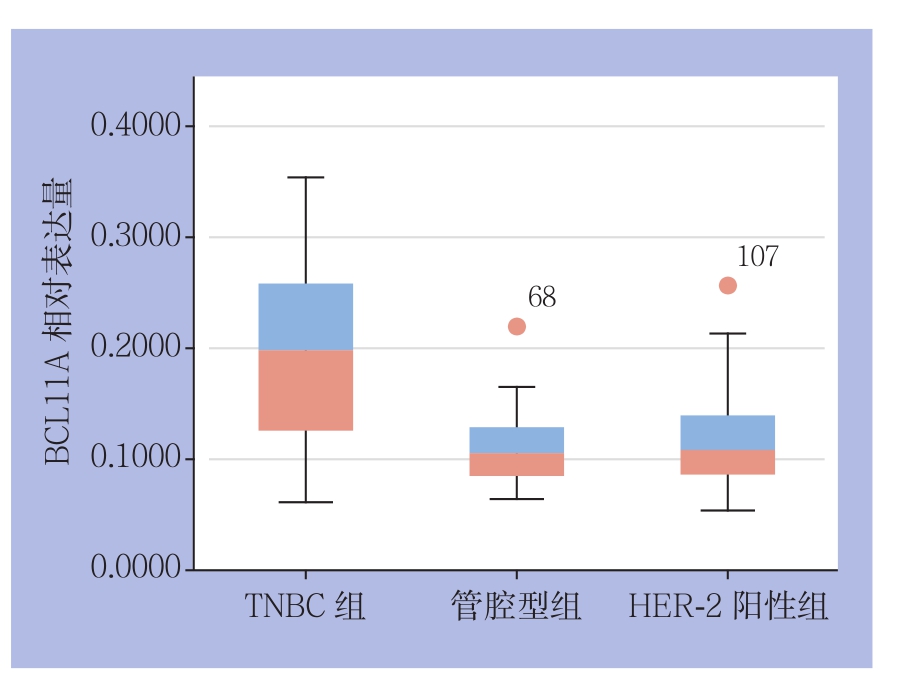
图2 BCL11A在3类乳腺癌组织中的表达量比较
Figure 2 Comparison of BCL11A expression levels among the three types of breast cancer tissues
2.2 BCL11A在TNBC组织中的表达临床病理特点之间的关系
在TNBC中,BCL11A的阳性表达与原发肿块大小明显有关(P<0.05)。BCL11A的阳性表达与患者年龄、淋巴结状态、Ki67、临床分期、组织学分级、乳腺癌家族史之间无明显关系(均P>0.05)(表2)。
表2 BCL11A表达与TNBC临床病理特征的关系[n(%)]
Table 2 Relations of BCL11A expression with the clinicopathologic features of TNBC [n (%)]

2.3 新辅助化疗对BCL11A表达水平的影响
对43例TNBC患者新辅助化疗前后的病理切片进行免疫组化分析,结果显示,BCL11A在化疗前组和化疗后组的阳性表达率分别为76.7%(33/43),55.8%(24/43),化疗前组BCL11A阳性表达率明显高于化疗后组(P=0.04);对u值进行定量分析,化疗前组u值明显大于化疗后组(P=0.03)(图3)(表3)。
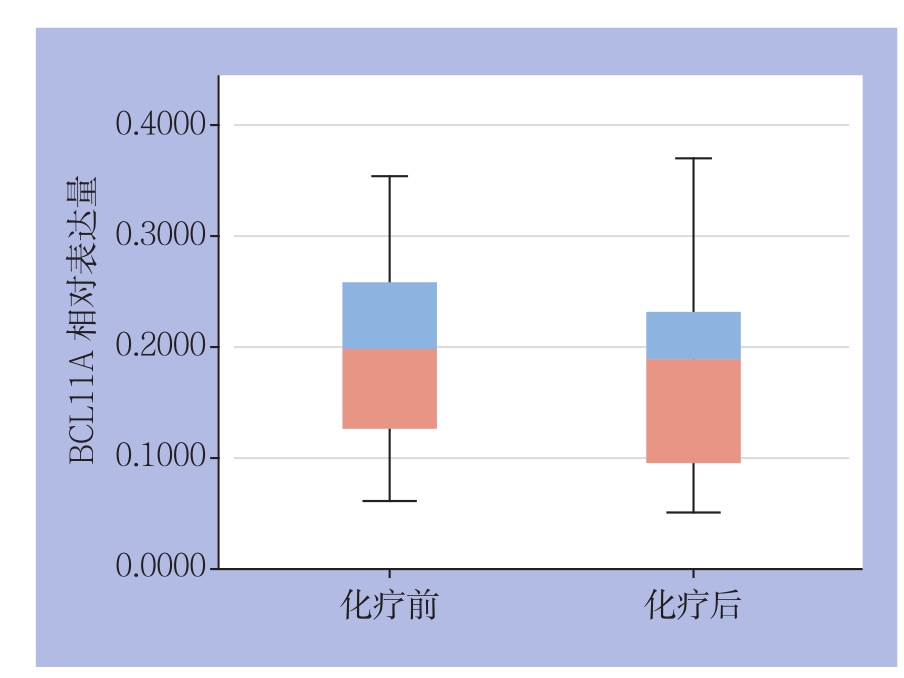
图3 TNBC组织新辅助化疗前后BCL11A表达量比较
Figure 3 Comparison of the BCL11A expression levels in TNBC tissue before and after neoadjuvant chemotherapy
表3 TNBC组织新辅助化疗前后BCL11A的表达情况(n=43)
Table 3 BCL11A expressions in TNBC tissue before and after neoadjuvant chemotherapy (n=43)

3 讨 论
TNBC最初在2005年10月提出,随后该名词出现在600多篇文章中[13],TNBC具有平均发病年龄小,组织学分级高,侵袭性强,细胞增殖率高,易复发及转移等特点,化疗是TNBC唯一有效的全身综合治疗手段[4-6]。本研究结果表明BCL11A在TNBC化疗前组的表达率明显高于非TNBC组,TNBC化疗前组、管腔型组、HER-2阳性组中BCL11A的阳性率分别为76.7%(33/43)、26.5%(13/49)、32.0%(16/50),与Khaled等[12]报道的BCL11A在TNBC,官腔型,HER-2阳性型中阳性率(分别为67%、12%、29%)基本一致。表明BCL11A不仅在欧美人的TNBC中高表达,在我国人群中的TNBC中也是高表达,且阳性率明显高于其他类型的乳腺癌。本研究进一步发现新辅助化疗后BCL11A阳性率下降,BCL11A在TNBC的表达与原发肿块的大小有关。
BCL11A基因,最早于2000年在T(2;14)(p16;q32.3)异位的慢性淋巴细胞白血病中发现[7],位于人类基因组的2p13位点,在物种间高度保守。研究[14]发现BCL11A能与靶基因启动子区的5'-GGCCGG-3'基序结合,影响组蛋白H3/H4的去乙酸化,从而抑制靶基因的转录,但可被逆转录入病毒结合。BCL11A主要表达于胎肝、骨髓、脑组织中,并在B淋巴细胞、早期T祖细胞、淋巴系祖细胞以及造血干细胞等造血组织中高表达,在B淋巴细胞发育增殖中起到重要的作用[9-10]。BCL11A是B细胞淋巴瘤/白血病(BCL)家族的一员,是一种原癌基因,而BCL11中另一成员BCL11B是一个抑癌基因,其起到增加抗DNA损伤的作用[15-16]。以前的研究报道,增强的BCL11A表达可以抑制P21诱导,P21蛋白可以抑制白血病细胞复制及延长细胞周期,但BCL11A过表达能够下调P21的活性,从而出现白血病细胞复制加强及白血病细胞周期缩短,同时研究表明BCL11A高表达提示急性髓系白血病患者预后较差[17]。Yin等[18]也报道BCL11A作为癌基因,在小鼠不存在NF1的情况下可能导致白血病,可能是通过抑制P21诱导,从而促进细胞生长。Khaled等[12]研究显示BCL11A高表达能促进乳腺上皮细胞的集落生成及激发肿瘤的形成,BCL11A在TNBC中同样是原癌基因。本研究同样发现TNBC患者的原发肿块大小与BCL11A的表达有关,临床资料说明BCL11A存在促进乳腺癌肿瘤组织生长中的作用,但其在乳腺癌中的具体机制需进一步探索。
本研究另一结果提示经过新辅助化疗后,TNBC中BCL11A的表达水平及表达率降低,新辅助化疗前后BCL11A表达水平的差异具有统计学意义(P<0.05)。新辅助化疗是乳腺癌治疗中的重要一环节,可通过新辅助化疗予以患者降期,从而使不可手术患者达到手术条件,并在一定程度上判断药物的敏感性[19-21]。新辅助化疗影响BCL11A表达的机制,现阶段暂时缺乏相关研究。主要有以下可能:有研究[22-24]表明,新辅助化疗后,Ki67及p53肿瘤标记物表达水平可能较化疗前有所下降,其主要原因是与化疗药物使肿瘤细胞增殖能力下降有关,即新辅助化疗影响了细胞增殖能力,从而降低了Ki67及p53的表达。研究[25]表明,BCL11A编码的蛋白在细胞增殖和抗衰亡等方面起作用。根据本研究结果推测,化疗可能影响了BCL11A编码蛋白对于细胞增殖的作用,从而导致化疗前后BCL11A蛋白表达的水平有所下降。另一种推测,由于肿瘤细胞异质性,在同一患者肿瘤组织中,BCL11A在不同肿瘤细胞中表达不尽相同,因化疗药物往往对于增殖能力强的癌细胞杀伤力更大,新辅助化疗清除了大量BCL11A表达阳性的癌细胞,从而BCL11A表达阳性的细胞比例相对降低。在后续研究中,笔者将进一步探索这种现象是否与化疗疗效及患者预后存在一定的相关性。
综上所述,BCL11A在TNBC中高水平表达,其表达水平受新辅助化疗影响,其代表的临床意义有待进一步深入研究。
参考文献
[1]Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumors[J]. Nature, 2000, 406(6797):747–752.
[2]Carey L, Winer E, Viale G, et al. Triple-negative breast cancer:disease entity or title of convenience?[J]. Nat Rev Clin Oncol, 2010,7(12):683–692. doi: 10.1038/nrclinonc.2010.154.
[3]Montagna E , Bagnardi V , Rotmensz N,et al. Outcome and Medial Presentation of Breast Cancer: European Institute of Oncology Experience.[J]. Clin Breast Cancer, 2015, 15(6):440–447.doi: 10.1016/j.clbc.2015.07.003.
[4]夏林玉, 徐卫云. 三阴性乳腺癌治疗的新进展[J]. 中国普通外科杂志, 2016, 25(5):741–746. doi:10.3978/j.issn.1005–6947.2016.05.020.Xia LY, Xu WY. Treatment of triple-negative breast cancer: resent progress[J]. Chinese Journal of General Surgery, 2016, 25(5):741–746. doi:10.3978/j.issn.1005–6947.2016.05.020.
[5]Darb-Esfahani S, Denkert C, Stenzinger A, et al. Role of TP53 mutations in triple negative and HER2-positive breast cancer treated with neoadjuvant anthracycline/taxane-based chemotherapy[J].Oncotarget, 2016, 7(42):67686–67698. doi: 10.18632/oncotarget.11891.
[6]Bakrania AK, Variya BC, Patel SS. Novel targets for paclitaxel nano formulations: Hopes and hypes in triple negative breast cancer[J]. Pharmacol Res, 2016, 111:577–591. doi: 10.1016/j.phrs.2016.07.023.
[7]Satterwhite E, Sonoki T, Willis TG, et al. The BCL11 gene family:involvement of BCL11A in lymphoid malignancies[J]. Blood, 2001,98(12):3413–3420.
[8]Xu L, Wu H, Wu X, et al. The expression pattern of Bcl11a, Mdm2 and Pten genes in B-cell acute lymphoblastic leukemia[J]. Asia Pac J Clin Oncol, 2017, doi: 10.1111/ajco.12690. [Epub ahead of print]
[9]Kuo TY, Hsueh YP. Expression of zinc finger transcription factor Bcl11A/Evi9/CTIP 1 in rat brain[J]. J Neurosci Res, 2007,85(8):1628–1636.
[10]Sankaran VG, Menne TF, Xu J, et al. Human fetal hemoglobin expression is regulated by the developmental stage—specific repressor BCL11A[J]. Science, 2008, 322(5909):1839–1842. doi:10.1126/science.1165409.
[11]Dong H, Shi P, Zhou Y, et al. High BCL11A Expression in Adult Acute Myeloid Leukemia Patients Predicts a Worse Clinical Outcome[J]. Clin Lab, 2017, 63(1):85–90 doi: 10.7754/Clin.Lab.2016.160614.
[12]Khaled WT, Choon Lee S, Stingl J, et al. BCL11A is a triplenegative breast cancer gene with critical functions in stem and progenitor cells[J]. Nat Commun, 2015, 6:5987. doi: 10.1038/ncomms6987.
[13]Brenton JD, Carey LA, Ahmed AA, et al. Molecular classification and molecular forecasting of breast cancer: ready for clinical application?[J]. J Clin Oncol, 2005, 23(29):7350–7360.
[14]Chen Z, Luo HY, Steinberg MH, et al. BCL11A represses HBG transcription in K562 cells[J]. Blood Cells Mol Dis, 2009,42(2):144–149. doi: 10.1016/j.bcmd.2008.12.003.
[15]Kamimura K, Ohi H, Kubota T, et al. Haploinsufficiency of Bcl11b for suppression of lymphomagenesis and thymocyte development[J].Biochem Biophys Res Commun, 2007, 355(2):538–542.
[16]Kamimura K, Mishima Y, Obata M, et al. Lack of Bcl11b tumor suppressor results in vulnerability to DNA replication stress and damages[J]. Oncogene, 2007, 26(40):5840–5850.
[17]Schmelz K1, Wagner M, Dörken B, et al. 5-Aza-2'-deoxycytidine induces p21WAF expression by demethylation of p73 leading to p53-independent apoptosis in myeloid leukemia[J]. Int J Cancer,2005, 114(5):683–695.
[18]Yin B, Delwel R, Valk PJ, et al. A retroviral mutagenesis screen reveals strong cooperation between Bcl11a overexpression and loss of the Nf1 tumor suppressor gene[J]. Blood, 2009, 113(5):1075–1085. doi: 10.1182/blood–2008–03–144436.
[19]Campbell JI, Yau C, Krass P, et al. Comparison of residual cancer burden, American Joint Committee on Cancer staging and pathologic complete response in breast cancer after neoadjuvant chemotherapy: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657)[J]. Breast Cancer Res Treat, 2017,doi: 10.1007/s10549–017–4303–8. [Epub ahead of print]
[20]Petruolo OA, Pilewskie M, Patil S, et al. Standard Pathologic Features Can Be Used to Identify a Subset of Estrogen Receptor-Positive, HER2 Negative Patients Likely to Benefit from Neoadjuvant Chemotherapy[J]. Ann Surg Oncol, 2017, doi:10.1245/s10434–017–5898–z. [Epub ahead of print]
[21]Matsuda N, Kida K, Ohde S, et al. Change in sonographic brightness can predict pathological response of triple-negative breast cancer to neoadjuvant chemotherapy[J]. Breast Cancer, 2017,doi: 10.1007/s12282–017–0782–z. [Epub ahead of print]
[22]Stroescu C, Dragnea A, Ivanov B, et al. Expression of p53,Bcl-2,VEGF,Ki67 and PCNA and prognostic significance in hepatocellular carcinoma[J]. J Gastrointestin Liver Dis, 2008,17(4):411–417.
[23]Sheri A, Smith IE, Hills M, et al. Relationship between IHC4 score and response to neo-adjuvant chemotherapy in estrogen receptorpositive breast cancer[J]. Breast Cancer Res Treat, 2017, doi:10.1007/s10549–017–4266–9. [Epub ahead of print]
[24]Tokuda E, Horimoto Y, Arakawa A, et al. Differences in Ki67 expressions between pre- and post-neoadjuvant chemotherapy specimens might predict early recurrence of breast cancer[J]. Hum Pathol, 2017, doi: 10.1016/j.humpath.2017.02.005. [Epub ahead of print]
[25]Yu Y, Wang J, Khaled W, et al. Bcl11a is essential for lymphoid development and negatively regulates p53[J]. J Exp Med, 2012,209(13):2467–2483. doi: 10.1084/jem.20121846.
