巨块型肝癌合并肝硬化时的手术抉择是很艰难的,面临着大出血及术后因残留肝体积不足而导致肝功能衰竭的可能。2012年德国Schnitzbauer等[1]首先提出一种全新的肝切除术式-联合肝脏分隔和门静脉结扎的二步肝切除术(associating liver partition and portal vein ligation for staged hepatectomy,ALPPS),为巨块型肝癌的手术切除提供了一种新的选择。其原理是通过选择性结扎一侧肝脏的门静脉主干,调整改变肝脏的入肝血流,从而促进余肝实质(future liver remnant,FLR)的再生,提高手术切除机会。其通过分二步实施手术的策略,先手术干预提升FLR,避免肝切除术后肝衰竭发生,再达到完整切除肝癌的目的。经典的ALPPS术都是2次开腹手术,并强调分离沟左右两侧肝实质的充分离断,且用无菌袋将肿瘤侧隔离。近年来,随着手术报道例数的增加,经典的ALPPS术因其具有创伤大、肿瘤易播散、手术风险大等缺点而备受争议。各种改良的ALPPS相继零星报道,如全腹腔镜ALPPS、微波消融ALPPS、射频消融ALPPS等,本组全部病例则尝试采用另一种改良方法:即第一次手术在全腹腔镜下完成,分离沟左右两侧肝实质的离断不要求过深,第2次手术在开腹下完成。笔者回顾性分析2014年4月—2016年4月在我院住院的12例原发性肝癌的临床资料及手术治疗情况,尝试探讨腹腔镜一期开腹二期ALPPS的安全性及疗效,现报告如下。
1 资料与方法
1.1 一般资料
本组患者共12例,男10例,女2例;年龄45~67岁,平均58.2岁。所有病患者均有乙型肝炎后肝硬化病史。AFP阳性10例。第1次术前生化检查:谷丙转氨酶平均为(44.1±5)U/L,谷草转氨酶平均为(128.9±13)U/L,总胆红素平均为(11±4.5)μmol/L,直接胆红素平均为(5.2±2.6)μmol/L,白蛋白平均为(31.4±6)g/L。12例术前均应用法国Intrasense公司MyrianXP-Liver软件行肝脏三维重建并测定剩余肝体积(FLR)及其占全肝体积比例(余肝体积/全肝体积),其平均值为(308±64)mL与(27±3.8)%,凝血酶原时间及凝血酶时间均正常。术前肝功能评级为Child B级。术前CT或MRI均发现肝脏巨大占位性病变(肿瘤直径>10 cm),其中占据右半肝7例,占据右三肝5例,无门脉癌栓及肝内播散。术前ICGR15>20%,均值为(23.4±1.5)%。术前诊断为原发性肝癌(巨块型),术前均未行介入及化疗。
1.2 手术方法
采用全身麻醉方式及术中控制性低中心静脉压技术。第1次手术为全腹腔镜ALPPS。采用德囯STORZE腹腔镜系统,患者取仰卧位,头高脚低,右侧腰部垫高。分别于剑突下、左锁骨中线、脐下、右锁骨中线、右腋前线处(5孔法)置入各镜鞘及手术器械,脐下为腹腔镜镜头放置孔。先切除胆囊,解剖右侧肝蒂,分离出右门静脉主干并绕线结扎(图1)。在左右半肝或者左内、左外叶之间以超声刀离断部分肝实质(深度3~4 cm)。离断沟内置入纤维蛋白凝胶或明胶海绵。术后予以护肝、白蛋白支持治疗,术后7~14 d复查肝功能及CT,评估余肝增生及体积大小(图2)。第2次手术为开腹手术,行扩大右半肝切除或右三肝切除(图3)。先分离粘连并显露肝门,第一肝门预置全肝血流阻断带。分别将右侧胆管、肝动脉及先前结扎过的门静脉切断并缝扎处理;沿离断沟完整离断肝实质,注意勿损伤肝后下腔静脉。仔细处理肝右静脉或肝中静脉,将左右肝脏完全劈离后再游离并切断右肝周围韧带,处理各肝短静脉后,将巨大肝癌连同右肝一并切除。
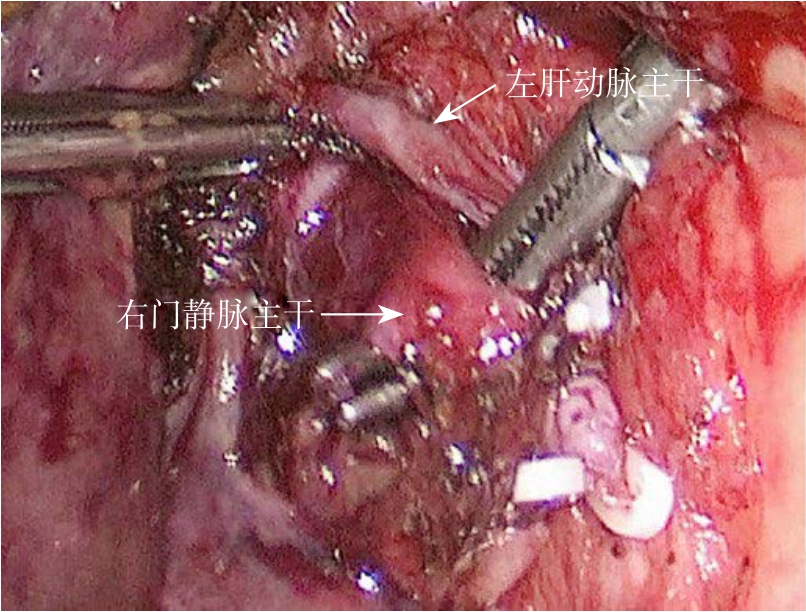
图1 腹腔镜下游离出右门静脉主干待结扎
Figure 1 Laparoscopic dissociation of the right portal vein for ligation
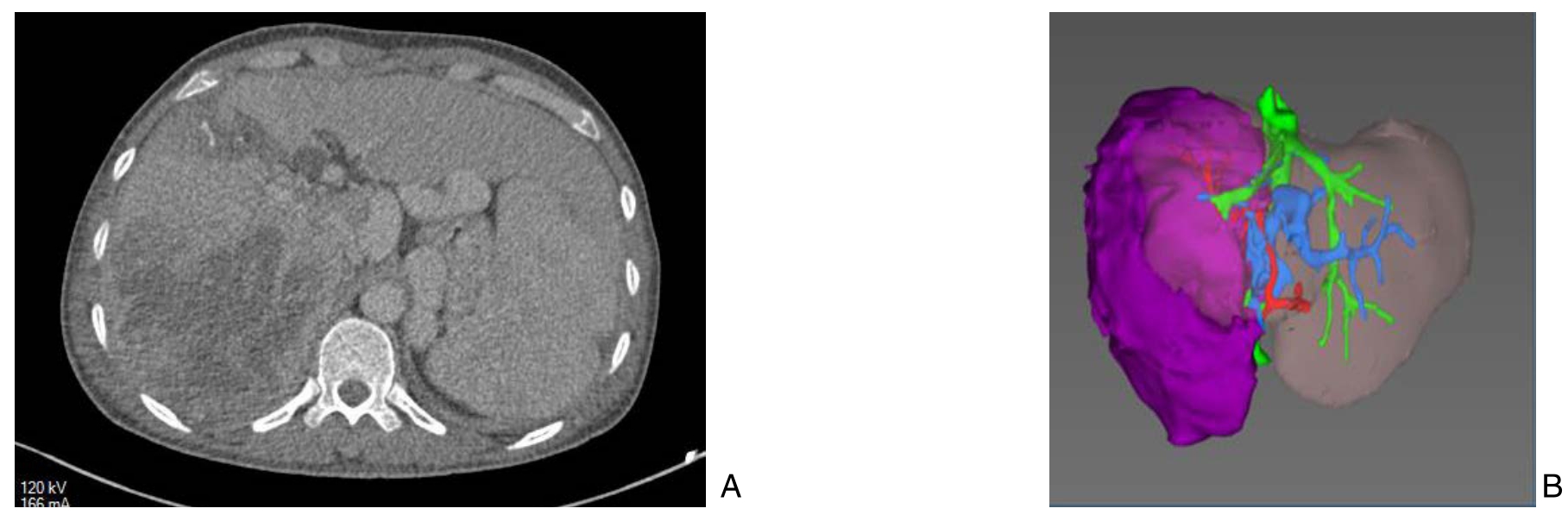
图2 第一期术后10 d影像资料
Figure 2 Imaging data 10 d after the first-stage operation
A:CT复查可见离断沟;B:三维重建(紫色代表肿瘤)示左外叶体积占比增大至46.8%(FLR为883 mL,全肝体积为1 886 mL)
A: CT scan showing an obvious dividing line; B: Three-demesional reconstruction (purple color for the tumor) showing the volume of left lateral lobe had increased to 46.8% of the total liver volume(883 mL for FLR and 1 886 mL for total estimated liver volume)
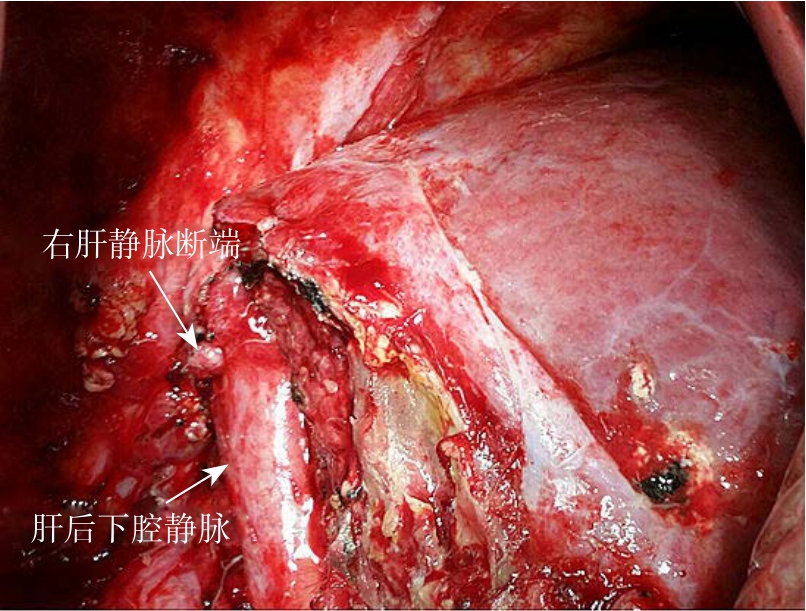
图3 二期右三肝切除术
Figure 3 Second stage right hepatic trisegmentectomy
1.3 统计学处理
所有变量资料的平均数计算均应用SPSS 16.0软件处理。
2 结 果
2.1 一期手术情况
第一期12例手术均在全腹腔镜下实施,其中1例在腹腔镜下离断肝实质中出血较多(500 mL)而及时中转开腹,平均手术时间(230±15)min,7~14 d后复查肝功能及术后ICGR15,所有患者的肝功能分级均由Child B级转为Child A级,FLR体积为(684±129)mL,占全肝体积比(56±7.7)%,ICGR15为(8.6±4.2)%,较术前明显降低(P<0.01)。术后肝功能得到明显改善,转氨酶及胆红素下降,白蛋白上升。2次手术的平均间隔时间为10.5(7~16)d(表1)。
2.2 二期手术情况
第二期手术为开腹手术,其中7例为扩大右半肝切除术,5例为右三肝切除术,术中平均失血量650(200~1200)mL,术中平均输血量为3.5(1.5~6)U。术后病理均为原发性肝细胞癌。
2.3 术后并发症
全组病例未发生大出血及肝功能衰竭等严重并发症。术后所有患者在一、二期手术后均出现低蛋白性腹水,经补白蛋白、利尿、护肝治疗后消失。3例患者出现一过性胆汁漏,经保守治疗后痊愈。患者术后平均住院时间为22.5 d。
表1 12例患者术前与一期术后FLR、ICGR15变化及与二期手术的间隔时间
Table 1 The changes in FLR and ICGR15 before and after the first-stage operation and time interval to the second-stage operation in the 12 patients
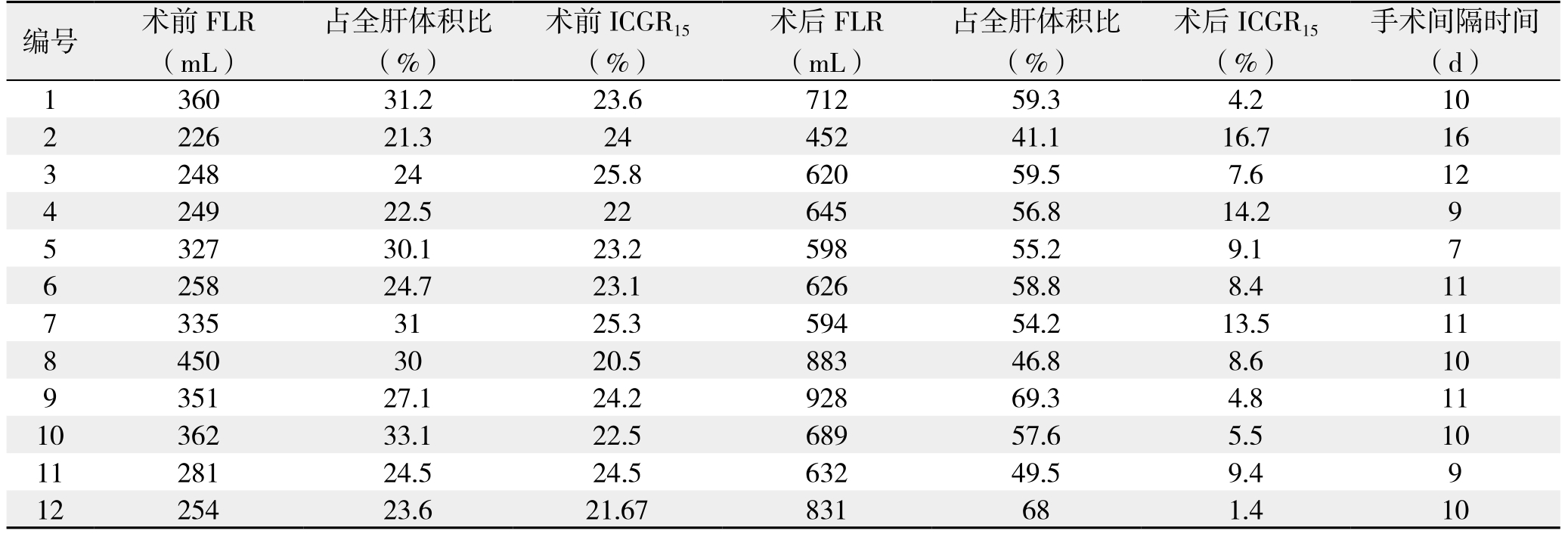
编号 术前FLR(mL)手术间隔时间(d)1 360 31.2 23.6 712 59.3 4.2 10 2 226 21.3 24 452 41.1 16.7 16 3 248 24 25.8 620 59.5 7.6 12 4 249 22.5 22 645 56.8 14.2 9 5 327 30.1 23.2 598 55.2 9.1 7 6 258 24.7 23.1 626 58.8 8.4 11 7 335 31 25.3 594 54.2 13.5 11 8 450 30 20.5 883 46.8 8.6 10 9 351 27.1 24.2 928 69.3 4.8 11 10 362 33.1 22.5 689 57.6 5.5 10 11 281 24.5 24.5 632 49.5 9.4 9 12 254 23.6 21.67 831 68 1.4 10占全肝体积比(%)术前ICGR15(%)术后FLR(mL)占全肝体积比(%)术后ICGR15(%)
2.4 术后随访
12例患者均成功回访,随访时间为6~30个月,其中7例患者随访时间超过1年,5例无瘤生存时间超过1年;3例分别于术后1、9、16个月死亡。其余患者尚在随访中。
3 讨 论
随着肝脏外科技术的发展,目前对肝癌的手术切除已无禁区,但全球肝癌的手术切除率仍然低于30%[2],其主要原因之一是因肿瘤体积过大,剩余肝实质无法耐受极量肝切除术,术后容易发生肝功能衰竭。2012年德国Schnitzbauer等[1]首先提出ALPPS手术可望针对性地解决余肝功能代偿不足的问题,为晚期肝癌的治疗提供了一种新的策略。主要适用于肿瘤巨大或者多个,余肝体积严重不足,不合并有门静脉主干癌栓、下腔静脉癌栓和远处转移的病例。基础研究表明在第一次术后7~13 d内未来剩余肝脏体积可急速提升到45%~78%左右,从而为第2次安全实施大范围肝切除术创造了有利机会[3-4,6]。国外大多应用于肝脏转移瘤手术[5-9],残肝质地良好,国内的则大部分为原发性肝癌,常常合并肝硬化,严重者可出现肝功能失代偿[10-13]。
对于合并肝硬化的Child B级患者实施大范围肝切除术是相对禁忌证,而且理论上要求FLR体积必须达到40%以上,即使实施ALPPS术也是如此。但是,由于ALPPS一期仅为门静脉结扎和部分肝实质离断,对肝脏损伤较小,术后患者的肝功能可能改善,因此ALPPS术对此类患者仍然是适应的[14]。本组患者也显示了患者的肝功能在ALPPS术后得到明显改善,转氨酶及胆红素下降,白蛋白上升,应该得益于剩余肝组织的代偿性增生。但是也有作者[15-16]认为,肝硬化程度是影响肝脏再生能力的重要因素,对合并高胆红素血症的肝硬化患者施行需要残肝发挥肝再生功能以保障疗效的ALPPS术式可能风险更高。笔者认为,对术前合并肝硬化的Child B级患者实施ALPPS仍需谨慎,除术前常规的抗病毒治疗及护肝治疗外,还强调离断肝实质的适度(深度3~4 cm),不必过分追求肝实质的充分离断,只要能达到刺激剩余肝实质的创伤-再生即可。若ALPPS-1后肝功能仍达不到Child A级,FLR达不到40%,则应放弃下一步的手术计划。本组患者第1次手术术前FLR体积仅为(27±3.8)%,ICGR15>20%,肝功能评级Child B级,按常规方法无法耐受巨块型肝癌切除手术,在完成一期术后的再次评估中,发现12例患者的残肝体积明显增大至(56±7.7)%,肝功能分级提升至Child A级,为第2次切除手术创造了条件。
ALPPS术备受争论的焦点是[4,15-18]:⑴ 对于晚期肝癌,开展此类手术的手术价值问题。⑵ ALPPS术的手术并发症较高,有文献[19-23]报道其病死率高达12%~23%。发生胆汁漏,伴严重感染的发生率高达20%~25%。⑶ 违背no touch技术的原则,甚至有学者称它为“all touch technique”。理论上术中癌细胞扩散风险很高[15]。因此,ALPPS术治疗晚期肝癌目前仍然属于探讨阶段,其有效性、安全性尚有待进一步观察[22-23]。2013年周俭等[10]报告成功在国内实施了1例ALPPS手术后,国内不同诊治中心纷纷开展这项新技术[10-12],但报道例数均为个位数,并对传统ALPPS尝试做了不同程度的改良,相继开展了腹腔镜ALPPS、微波消融ALPPS、射频消融ALPPS及机器人辅助ALPPS等,均取了一定疗效[24-28]。
经典的开腹ALPPS具有创伤大和2次手术的固有局限性,一期手术中肝脏离断和门静脉右支结扎可能导致严重的炎性反应和术后粘连,将增加第二期手术的难度和风险[16,20]。故采用恰当有效的损伤控制手段将有助于保证术后余肝实质的扩增能力,降低第二期手术的难度和风险[29]。针对如何减少创伤和提高手术安全,笔者所在团队尝试应用腹腔镜技术相继完成了一期腹腔镜、二期开腹ALPPS术12例。笔者的体会是,相比于经典ALPPS,第1次实施腹腔镜手术有利于减少术后粘连,减轻术后腹水程度,缩短手术间隔时间,为术后肝功能早期恢复及第2次手术顺利实施创造条件。由于是在腹腔镜下操作,杜绝了“all touch technique”的可能,减少了医源性肿瘤播散的发生。经典ALPPS术中容易发生大出血,其发生率为8%~14%[16-17,30],尤其在合并肝硬化时,大出血主要发生在离断肝实质沟和行大范围肝切除时。笔者所在团队对此做了相应改进,在第1次腹腔镜手术时,除常规采用低中心静脉压技术外,离断左右肝实质强调适度(深度3~4 cm),既可以避免发生大出血,又可以防止IV段缺血坏死。本组术中平均失血量650(200~1 200)mL,明显低于经典ALPPS的平均失血量1.5 L[2,30-31]。相比于全腹腔镜ALPPS,笔者认为第2次手术采用开腹手术更为安全。第2次手术术中可见组织水肿明显,会导致腹腔镜下解剖困难,尤其对巨大肝癌临近第一肝门者。
本组除1例在一期手术时中转开腹外,其他11例一期均在全腹腔镜下完成,ALPPS术后剩余肝组织代偿性增生明显,肝功能分级也相应好转,且术后的严重并发症明显低于同类报道[32-33],未发生大出血病例,围手术期病死率为0。因此,笔者认为腹腔镜一期开腹二期ALPPS术治疗巨块型肝癌是可行的、安全的。但由于病例数尚少,且随访时间还不够,其长期有效性尚待评估。
参考文献
[1] Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings[J]. Ann Surg, 2012, 255(3):405–414. doi: 10.1097/SLA.0b013e31824856f5.
[2] Zhou Y, Lei X, Wu L, et al.Outcomes of hepatectomyfor noncirrhotic hepatocellular carcinoma: a systematic review[J]. Surg Oncol, 2014, 23(4):236–242. doi: 10.1016/j.suronc.2014.11.001.
[3] de Santibañes E, Clavien PA. Playing Play-Doh to prevent postoperative liver failure:the“ALPPS”approach[J]. Ann Surg,2012, 255(3):415–417. doi: 10.1097/SLA.0b013e318248577d.
[4] Wei W, Zhang T, Zafarnia S, et al. Establishment of a rat model:Associating liver partition with portal vein ligation for staged hepatectomy[J].Surgery,2016, 159(5):1299–1307.doi: 10.1016/j.surg.2015.12.005.
[5] 曹君, 陈亚进. 联合肝脏离断和门静脉结扎二步肝切除术几个焦点问题[J]. 中国实用外科杂志, 2014, 34(8):713–716. doi: 10.7504/CJPS.ISSN1005–2208.2014.08.12.Cao J, Chen YJ. Some hot topics on associating liver partition and portal vein ligation for staged hepatectomy[J]. Chinese Journal of Practical Surgery, 2014, 34(8):713–716. doi: 10.7504/CJPS.ISSN1005–2208.2014.08.12.
[6] Clavien PA, Petrowsky H, DeOliveira ML, et al. Strategies for safer liver surgery and partial liver transplantation[J]. N Engl J Med,2007, 356(15):1545–1559.
[7] Alvarez FA, Ardiles V, Sanchez Claria R, et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS):tips and tricks[J]. J Gastrointest Surg, 2013, 17(4):814–821. doi:10.1007/s11605–012–2092–2.
[8] Vennarecci G, LaurenziA, Levi Sandri GB, et al. The ALPPS procedure for hepatocellular carcinoma[J]. Eur J SurgOncol, 2014,40(8):982–988. doi: 10.1016/j.ejso.2014.04.002.
[9] Oldhafer KJ, Donati M, Jenner RM, et al. ALPPS for Patients with Colorectal Liver Metastases:EffectiveLiver Hypertrophy, but Early Tumor Recurrence[J]. World J Surg, 2014, 38(6):1504–1509.doi:10.1007/s00268–013–2401–2.
[10] 周俭, 王征, 孙健, 等. 联合肝脏离断和门静脉结扎的二步肝切除术[J]. 中华消化外科杂志, 2013, 12(7):485–489. doi: 10.3760/cma.j.issn.1673–9752.2013.07.002.Zhou J, Wang Z, Sun J, et al. Associating liver partition and portal vien ligation for staged hepatectomy[J]. Chinese Journal of Digestive Surgery, 2013, 12(7):485–489. doi: 10.3760/cma.j.issn.1673–9752.2013.07.002.
[11] 王志明, 陶一明, 黄云, 等. 联合肝脏离断和门静脉切断二步肝切除术在肝炎后肝硬化肝癌中的应用[J]. 中国普通外科杂志,2014, 23(7):867–872. doi: 10.7659/j.issn.1005–6947.2014.07.001.Wang ZM, Tao YM, Huang Y, et al. Associating liver partition and portal vein ligation for staged hepatectomy procedure in treatment of hepatocellular carcinoma with post-hepatitic cirrhosis[J]. Chinese Journal of General Surgery, 2014, 23(7):867–872. doi: 10.7659/j.issn.1005–6947.2014.07.001.
[12] 杨扬, 程张军, 周家华, 等. 联合肝脏离断和门静脉结扎的二步肝切除术治疗肝硬化巨大肝癌2例报告并文献复习[J]. 中国普通外科杂志, 2016, 25(7):965–972.doi: 10.3978/j.issn.1005–6947.2016.07.006.Yang Y, Cheng ZJ, Zhou JH, et al. Associating liver partition and portal vein ligation for staged hepatectomy in treatment of massive liver cancer with cirrhosis: a report of 2 cases and literature review[J]. Chinese Journal of General Surgery, 2016, 25(7):965–972.doi: 10.3978/j.issn.1005–6947.2016.07.006.
[13] Dai WD, Hu JX, Miao XY, et al. Intrahepatic Glissonian access for mesohepatectomy in cirrhotic patients[J]. Hepatogastroenterology,2008, 55(85):1153–1157.
[14] Serenari M, Zanello M, Schadde E, et al. Importance of primary indication and liver function between stages: results of a multicenter Italian audit of ALPPS 2012–2014[J]. HPB (Oxford).2016,18(5):419–27.
[15] 刘允怡, 刘晓欣. 对“联合肝脏离断和门静脉结扎的二步肝切除术”的述评[J]. 中华消化外科杂志, 2013, 12(7):481–484. doi:10.3760/cma.j.issn.1673–9752.2013.07.001.Liu YY, Liu XX. Comments on Associating liver partition and portal vein ligation for staged hepatectomy[J]. Chinese Journal of Digestive Surgery, 2013, 12(7):481–484. doi: 10.3760/cma.j.issn.1673–9752.2013.07.001.
[16] Lau WY, Lai EC, Lau SH. Associating liver partition and portal vein ligation for staged hepatectomy: the current role and development[J]. HepatobiliaryPancreat Dis Int, 2017, 16(1):17–26.
[17] Wigmore SJ.ALPPS: The argument against[J].Eur J SurgOncol,2017,43(2):249–251. doi: 10.1016/j.ejso.2016.11.009.
[18] Lodge JP. ALPPS: The argument for[J].Eur J SurgOncol,2017,43(2):246–248.doi: 10.1016/j.ejso.2016.11.007.
[19] Kokudo N, Shindoh J. How can we safely climb the ALPPS?[J].Updates Surg, 2013, 65(3):175–177. doi: 10.1007/s13304–013–0215–2.
[20] Aloia TA, Vauthey JN. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): what is gained and what is lost?[J]. Ann Surg, 2012, 256(3):e9. doi: 10.1097/SLA.0b013e318265fd3e.
[21] Sotiropoulos GC, Kouraklis G. The ALPPS procedure for extended indications in liver surgery: an old finding applied in surgical oncology[J]. Ann Surg, 2013, 285(6):e26. doi: 10.1097/SLA.0b013e3182942e4a.
[22] Figueras J, Belghiti J. The ALPPS approach: should we sacrifice basic therapeutic rules in the name of innovation[J]. World J Surg,2014, 38(6):1520–1521.doi: 10.1007/s00268–014–2540–0.
[23] Oldhafer KJ, Stavrou GA, van Gulik TM, et al.ALPPS--Where Do We Stand, Where Do We Go?: Eight Recommendations From the First International Expert Meeting[J].Ann Surg, 2016, 263(5):839–841. doi: 10.1097/SLA.0000000000001633.
[24] 曹君, 张红卫, 张磊, 等. 腹腔镜辅助联合肝脏离断和门静脉结扎二步肝切除术治疗原发性肝癌可行性研究[J]. 中国实用外科杂志, 2014, 34(1)77–80.Cao J, Zhang HW, Zhang L. et al. Laparoscopic-assisted associating liver partition and portal vein ligation for staged hepatectomy for primary liver cancer[J]. Chinese Journal of Practical Surgery, 2014,34(1)77–80.
[25] Gringeri E, Boetto R, D 'Amico FE, et al. Laparoscopic microwave ablation and portal vein ligation for staged hepatectom y (LAPS):a minim ally invasive first-step approach[J]. Ann Surgy, 2015,261(2):42–43. doi: 10.1097/SLA.0000000000000606.
[26] Gall TM, Sodergren MH, Frampton AE, et al. Radio-frequencyassisted Liver Partition with Portal vein ligation (RALPP) for liver regeneration[J]. Ann Surg, 2015, 261(2):e45–46. doi: 10.1097/SLA.0000000000000607.
[27] Hong de F, Zhang YB, Peng SY, et al.Percutaneous Microwave Ablation Liver Partition and Portal Vein Embolization for Rapid Liver Regeneration: A Minimally Invasive First Step of ALPPS for Hepatocellular Carcinoma[J].Ann Surg, 2016, 264(1):e1–2. doi:10.1097/SLA.0000000000001707.
[28] Vicente E, Quijano Y, Ielpo B, et al.FirstALPPSprocedure using a total robotic approach[J].Surg Oncol,2016, 25(4):457. doi: 10.1016/j.suronc.2015.10.001.
[29] 朱化刚. 术前肝脏储备功能的判断与安全肝切除量[J]. 肝胆外科杂志, 2005, 13(6):406–409.doi: 10.3969/j.issn.1006–4761.2005.06.003.Zhu HG. Preoperative assessment of liver functional reserve and safe resection volume[J]. Journal of Hepatobiliary Surgery, 2005,13(6):406–409.doi: 10.3969/j.issn.1006–4761.2005.06.003.
[30] Tanaka K, Endo I. ALPPS: short-term outcome and functional changes in the future liver remnant[J]. Ann Surg, 2015, 262(2):e88–89. doi: 10.1097/SLA.0000000000000665.
[31] Vivarelli M, Vincenzi P, Montalti R, et al. ALPPS Procedure for Extended Liver Resections: A Single Centre Experience and a Systematic Review[J].PLoS One, 2015, 10(12):e0144019. doi:10.1371/journal.pone.0144019.
[32] Andriani OC. Long-term results with associating liver partition and portal vein ligation for staged hepatectomy (ALPPS)[J]. Ann Surg,2012, 256(3):e5. doi: 10.1097/SLA.0b013e318265fbbe.
[33] Schadde E, Hernandez-Alejandro R, Lang H,et al.ALPPS Offers a Better Chance of Complete Resection in Patients with Primarily Unresectable Liver Tumors. Results of a Multicentre Analysis:Reply[J]. World J Surg, 2015, 39(7):1850–1851. doi: 10.1007/s00268–015–2964–1.