在大多数肝脏手术中,如肝移植,规则肝叶切除等,需分次分段阻断肝脏血流控制术中出血,以达到减少出血,同时减少其潜在的副损伤的目的。但该过程可造成肝脏的缺血再灌注损伤,引起肝细胞功能失调[1-2]。大量研究[3-5]表明,肝缺血再灌注损伤常造成肝窦内细胞损伤,从而诱导活性氧自由基富集,释放促炎症反应细胞因子,引起肝细胞坏死,也会引起肝细胞凋亡及自噬,表现出肝功能异常[6]。因此肝缺血再灌注损伤的防治一直是肝脏外科的重要研究领域。已有研究[7]证实中药单体苦参碱(Sophocarpidine,matrine)具有抗炎、抗氧化、保肝等作用,对缺血再灌注损伤引起的炎症反应、细胞凋亡、自噬及抗癌等可能有重要的调节作用[8-9]。另有研究[10-11]发现,苦参碱对肝缺血再灌注损伤引起的炎症反应、肝纤维化及肝细胞异常凋亡具有明显的抑制作用。但苦参碱抑制肝缺血再灌注损伤的机制尚未完全阐明。本研究在建立大鼠肝缺血再灌注损伤模型的基础上,观察苦参碱对肝脏缺血再灌注后的保护作用及相关机制。
1 材料与方法
1.1 实验材料
健康成年雄性SD大鼠,清洁级,40只,9~12周龄,体质量200~250 g,由中南大学实验动物学部提供。苦参碱(M5319)购于美国Sigma公司。丙氨酸转氨酶(ALT)检测试剂盒、天冬氨酸转氨酶(AST)检测试剂盒、TNF-α ELISA试剂盒和IL-1β ELISA试剂盒均由南京建成生物工程研究所提供;TUNEL染色试剂盒由美国Promega公司生产;肿瘤坏死因子相关的凋亡诱导配体(TRAIL)、BAX、激活型caspase-3(cleaved caspase-3,Asp175)及内参β-actin单克隆抗体均为英国Abcam公司产品。
1.2 实验方法
1.2.1 动物分组 40只SD大鼠随机均分为4组:⑴ 假手术组;⑵ 肝缺血再灌注损伤模型组(模型组);⑶ 低剂量苦参碱(25 mg/kg)预处理+肝缺血再灌注损伤模型组(低剂量苦参碱组);⑷ 高剂量苦参碱(50 mg/kg)预处理+肝缺血再灌注损伤模型组(高剂量苦参碱组)。
1.2.2 动物处理 术前12 h禁食,自由饮水,经腹腔注射4%戊巴比妥钠(40 mg /kg)麻醉,固定,常规消毒。取上腹正中切口入腹,低、高剂量苦参碱组大鼠在夹闭门静脉和肝动脉前30 min,经门静脉主干注入各自剂量的苦参碱溶液,假手术组与模型组大鼠则以相同的方式注入等体积的生理盐水;30 min后,模型组与低、高剂量苦参碱组大鼠用无创血管夹阻断肝左、中叶脉管,造成约70%肝脏缺血,60 min后放开血管夹,恢复阻断区血供再灌注120 min,建立肝切除肝缺血再灌注模型[12],并切除未经缺血处理的肝叶,约占全肝30%。假手术组仅行开腹手术,不行肝切除及肝缺血等处理。
1.2.3 标本处理 再灌注120 min后,采集各组大鼠肝上下腔静脉血,常温1200 r/min离心10 min,取血清保存待测;处死各组动物,切取右上叶肝组织,以中性福尔马林溶液固定,常规石蜡切片后用于组织病理学观察,以及肝细胞凋亡检测;另取5 g肝组织破碎离心后用于Western blot检测。
1.2.4 血清学指标检测 大鼠血清ALT、AST含量检测按照ALT、AST试剂盒说明进行,大鼠血清TNF-α、IL-1β的含量分别采用各自ELISA试剂盒检测。
1.2.5 组织病理学检测 HE染色后光镜下观察肝脏组织病理学的改变。
1.2.6 TUNEL法检测肝细胞凋亡 按试剂盒说明进行凋亡检测,DAB显色,苏木素复染。在显微镜下观察、计数、拍照。评判标准:阳性细胞核和细胞碎片呈深浅不一的棕黄色。每张切片随即选取10个高倍视野,计数凋亡细胞。凋亡指数(apoptosis index,AI)=视野内凋亡细胞数/视野内肝细胞数×100%。
1.2.7 Western blot检测 新鲜肝脏组织采用RIPA裂解液冰上完全裂解,12 000 r/min,4 ℃离心15 min,取上清收集总蛋白。5×上样缓冲液混合煮沸5 min。垂直板电泳分离条带,转膜,分别加入TRAIL、BAX、cleaved caspase-3单克隆抗体(一抗1:1 000)4 ℃孵育过夜,洗膜后用辣根过氧化物酶(HRP)标记的二抗(1:5 000)孵育2 h,利用化学发光仪曝光,采集目标蛋白条带。Western blot图像灰度值采用Quantity One 2.4软件分析。
1.3 统计学处理
采用SPSS 18.0软件处理数据,各组间比较采用单因素方差分析,两两比较采用q检验。P<0.05为差异有统计学意义。
2 结 果
2.1 各组转氨酶与炎症因子水平
血清学检测结果显示,与对照组比较,其余各组血清ALT、AST、TNF-α、IL-1β水平均明显升高(均P<0.05),但低、高剂量苦参碱组以上指标的升高程度均低于模型组(均P<0.05),且高剂量苦参碱组以上指标升高程度均低于低剂量苦参碱组(均P<0.05)(表1)。
表1 各组转氨酶与炎症因子水平比较(n=10, ±s)
±s)
Table 1 Comparison of the levels of transaminases and in fl ammatory factors among groups (n=10,  ±s)
±s)

注:1)与假手术组比较,P<0.05;2)与模型组比较,P<0.05;3)与低剂量苦参碱组比较,P<0.05
Note: 1) P<0.05 vs. sham operation group; 2) P<0.05 vs. model group; 3) P<0.05 vs. low dose matrine group
组别 ALT(U/L) AST(U/L) TNF-α(ng/L) IL-1β(ng/L)假手术组 49.3±5.7 150.1±11.4 2.11±0.47 1.37±0.25模型组 555.6±46.11) 796.1±57.21) 14.22±1.401) 7.64±0.831)低剂量苦参碱组 267.4±30.81),2) 445.7±37.81),2) 10.37±0.981),2) 4.92±0.941),2)高剂量苦参碱组 197.4±33.51),2),3) 408.9±44.41),2),3) 8.32±1.741),2),3) 2.55±1.111),2),3)
2.2 各组肝缺血再灌注损后肝组织病理学变化
光学显微镜下可见,假手术组肝组织无病理改变,模型组肝细胞肿胀、肝细胞空泡变性和点状坏死,大量炎性细胞浸润;低、高剂量苦参碱组肝组损伤程度及范围均明显小于模型组,表现为组织结构尚清楚,肝细胞轻、中度浊肿,点状嗜酸性变性,轻度肝细胞淤胆,少量炎性细胞浸润,且高剂量苦参碱组肝组织损伤程度轻于低剂量苦参碱组(图1)。

图1 各组肝组织病理学检测(HE×200)
A:假手术组;B:模型组;C:低剂量苦参碱组:D:高剂量苦参碱组
Figure 1 Histopathological examination in each group (HE×200)
A: Sham operation group; B: Model group; C: Low dose matrine group; D: High dose matrine group
2.3 各组缺血再灌注后的肝细胞凋亡情况
TUNEL染色结果观察显示,假手术组仅有少量的凋亡阳性细胞,模型组可见大量胞核黄染的阳性细胞,2个剂量苦参碱组阳性细胞明显少于模型组,高剂量苦参碱组阳性细胞减少数量更为明显;比较各组的AI,差异均有统计学意义(均P<0.05)(图2-3)。

图2 各组TUNEL染色结果(×200)
A:假手术组;B:模型组;C:低剂量苦参碱组:D:高剂量苦参碱组
Figure 2 TUNEL staining results in each group (×200)
A: Sham operation group; B: Model group; C: Low dose matrine group; D: High dose matrine group

图3 各组AI比较
注:1)与假手术组比较,P<0.05;2)与模型组比较,P<0.05;3)与低剂量苦参碱组比较,P<0.05
Figure 3 Comparison of AI among groups
Note: 1) P<0.05 vs.sham operation group; 2) P<0.05 vs. model group;3) P<0.05 vs. low dose matrine group
2.4 Western blot检测结果
Western blot结果显示,各组肝组织TRAIL、BAX、cleaved caspase-3蛋白的表达水平均明显高于假手术组(均P<0.05),其中模型组以上蛋白的上调最为明显,2个剂量苦参碱组以上蛋白上调程度均明显低于模型组,且高剂量苦参碱组上调程度低于低剂量苦参碱组(均P<0.05)(图4-5)。

图4 各组Western blot检测结果
Figure 4 Western blot results of each group
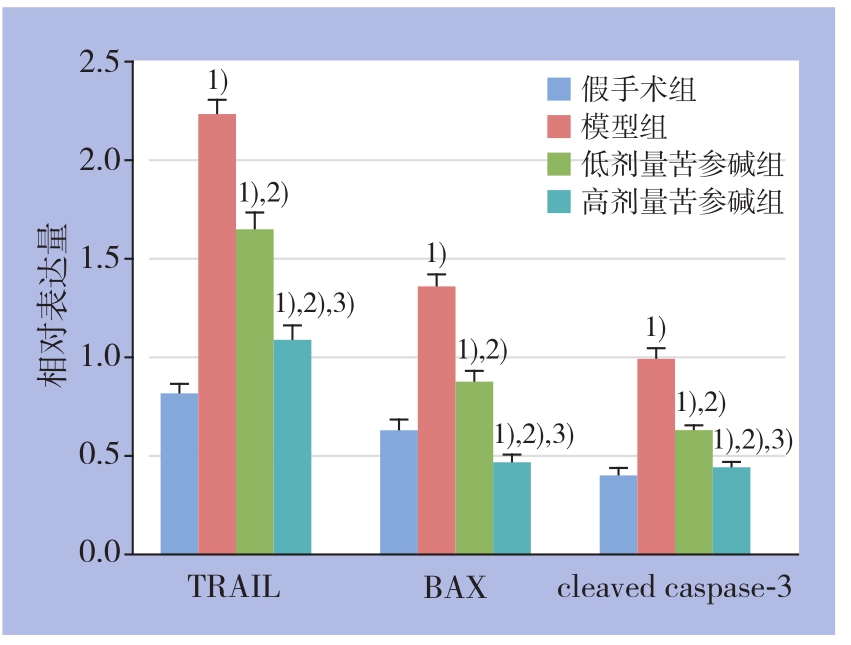
图5 各组各蛋白相对表达量比较
注:1)与假手术组比较,P<0.05;2)与模型组比较,P<0.05;3)与低剂量苦参碱组比较,P<0.05
Figure 5 Comparison of the relative protein expression levels among groups
Note: 1) P<0.05 vs. sham operation group; 2) P<0.05 vs. model group; 3) P<0.05 vs. low dose matrine group
3 讨 论
中药单体苦参碱具有抗炎、抗氧化、抗肿瘤、免疫抑制和保肝等作用[7]。已用于临床治疗,主要用于治疗慢性乙型病毒性肝炎[8],对多种原因引起的肝损伤及肝纤维化均有较好的治疗作用[8-9]。本研究通过大鼠肝缺血再灌注损伤模型,观察苦参碱的干预作用,结果发现,苦参碱对大鼠肝缺血再灌注损伤具有明显的保护作用,表现为血清转氨酶水平、炎症介质水平较模型组明显降低,肝组织病理损伤明显减轻,且呈明显的剂量依懒性。
为进一步探讨苦参碱抑制肝缺血再灌注损伤的机制,本研究检测了各组再灌注后肝细胞的凋亡情况,结果显示,苦参碱能明显减少缺血再灌注后肝组织的肝细胞凋亡。细胞凋亡是动物界及人类重要的细胞死亡模式,受多种因素的调控。TRAIL是近年来发现的新的TNF家族成员[13],TRAIL参与T细胞、NK细胞对集体的免疫监视,阻止肿瘤细胞转移,因此在肿瘤治疗方面备受关注。另外发现,TRAIL通过结合靶细胞相应的受体,发挥凋亡诱导作用。诸多研究证实,TRAIL对多种实体肿瘤细胞存在杀伤作用,如NF-κB可诱导TRAIL信号调节肝癌细胞凋亡[14],肿瘤免疫耐受过程中,TRAIL起重要的调节作用,许多海洋药类可通过TRAIL途径调节细胞凋亡[15]。近年越来越多的证据显示,在正常人细胞中,TRAIL具有调节细胞凋亡的作用。梁艳等[16]研究发现,TRAIL可诱导正常人肝细胞线粒体损伤,促进肝细胞凋亡,而且人角质细胞凋亡可通过TRAIL途径[17]。本研究结果显示,大鼠肝缺血再灌注后肝组织TRAIL蛋白表达明显升高,表明TRAIL途径在大鼠缺血再灌注引起的肝细胞凋亡过程中起重要作用。
TRAIL途径可通过诱导促凋亡蛋白BAX的表达,而BAX的激活可抑制肝细胞线粒体转膜能力,进而释放细胞色素C,促进凋亡关键因子caspase-3[18],最终引起细胞凋亡[17,19-21]。本研究证实,大鼠肝缺血再灌注后BAX蛋白以及激活型caspase-3的表达均明显上调,可见,在大鼠肝缺血再灌注损伤中,TRAIL-BAX-caspase-3途径的活化是肝细胞凋亡的重要原因。苦参碱预处理同样明显降低了缺血再灌注后肝组织BAX蛋白的表达以及激活型caspase-3水平。由此可有推测,苦参碱可能是通过抑制缺血再灌注后TRAIL的表达,进而减少了BAX与caspase-3的活化,减轻大鼠肝缺血再灌注后肝细胞的凋亡,从而有效抑制肝缺血再灌注损伤。
总之,苦参碱对大鼠肝缺血再灌注损伤有明显的抑制作用,本研究初步探讨了其作用机制,认为其抑制TRAIL-BAX-caspase-3途径引起的肝细胞凋亡可能是重要的作用机制之一。因而,进一步阐明其作用机制,并将其进一步开发利用,将有望对临床肝缺血再灌注损伤的防治提供有效手段。
参考文献
[1] Liu A, Jin H, Dirsch O, et al. Release of danger signals during ischemic storage of the liver: a potential marker of organ damage?[J]. Mediators In fl amm, 2010, 2010:436145. doi: 10.1155/2010/436145.
[2] 李玲, 傅华, 李汝泓, 等. 氟比洛酚酯对肝叶切除患者肝缺血再灌损伤保护作用的研究[J] .中国普通外科杂志, 2016, 25(12):1810–1814. doi:10.3978/j.issn.1005–6947.2016.12.024.Li L, Fu H, Li RH, et al. Study on the protective effect of flurbiprofen axetil on hepatic ischemia reperfusion injury in hepatectomy patients[J]. Chinese Journal of General Surgery, 2016,25(12):1810–1814. doi:10.3978/j.issn.1005–6947.2016.12.024.
[3] Kloek JJ, Maréchal X, Roelofsen J, et al. Cholestasis is associated with hepatic microvascular dysfunction and aberrant energy metabolism before and during ischemia-reperfusion[J]. Antioxid Redox Signal, 2012, 17(8):1109–1123. doi: 10.1089/ars.2011.4291.
[4] Hao BB, Pan XX, Fan Y, et al. Oleanolic acid attenuates liver ischemia reperfusion injury by HO-1/Sesn2 signaling pathway[J].Hepatobiliary Pancreat Dis Int, 2016, 15(5):519–524.
[5] 魏来, 陈文雁, 邹毅, 等. 硫化氢抑制自噬减轻肝硬化大鼠肝脏缺血再灌注损伤[J]. 中国普通外科杂志, 2016, 25(1):97–102.doi:10.3978/j.issn.1005–6947.2016.01.015.Wei L, Chen WY, Zou Y, et al. Protective effect of hydrogen sul fi de against hepatic ischemia-reperfusion injury in cirrhotic rats by autophagy inhibition[J]. Chinese Journal of General Surgery, 2016,25(1):97–102. doi:10.3978/j.issn.1005–6947.2016.01.015.
[6] Shen M, Lu J, Dai W, et al. Corrigendum to “Ethyl Pyruvate Ameliorates Hepatic Ischemia-Reperfusion Injury by Inhibiting Intrinsic Pathway of Apoptosis and Autophagy“[J]. Mediators In fl amm, 2016, 2016:5434275.
[7] Liou CJ, Lai YR, Chen YL, et al. Matrine Attenuates COX-2 and ICAM-1 Expressions in Human Lung Epithelial Cells and Prevents Acute Lung Injury in LPS-Induced Mice[J]. Mediators Inflamm,2016, 2016:3630485. doi: 10.1155/2016/3630485.
[8] Pu J, Fang FF, Li XQ, et al. Matrine Exerts a Strong Anti-Arthritic Effect on Type II Collagen-Induced Arthritis in Rats by Inhibiting In fl ammatory Responses[J]. Int J Mol Sci, 2016, 17(9). pii: E1410.doi: 10.3390/ijms17091410.
[9] Ma K, Huang MY, Guo YX, et al. Matrine-induced autophagy counteracts cell apoptosis via the ERK signaling pathway in osteosarcoma cells[J]. Oncol Lett, 2016, 12(3):1854–1860.
[10] Feng Y, Ying HY, Qu Y, et al. Novel matrine derivative MD-1 attenuates hepatic fi brosis by inhibiting EGFR activation of hepatic stellate cells[J]. Protein Cell, 2016, 7(9):662–672. doi: 10.1007/s13238–016–0285–2.
[11] 朱骏, 朱江, 唐发兵, 等. 苦参碱对大鼠肝脏缺血/再灌注损伤后肝功能和TNF-α的影响[J]. 中国临床研究, 2015, 28(9):1147–1150.doi: 10.13429/j.cnki.cjcr.2015.09.007.Zhu J, Zhu J, Tang FB, et al. In fl uence of matrine on liver functions and TNF-α level following liver ischemic reperfusion injury in rats[J]. Chinese Journal of Clinical Research, 2015, 28(9):1147–1150. doi: 10.13429/j.cnki.cjcr.2015.09.007.
[12] Hines IN, Hoffman JM, Scheerens H, et al. Regulation of postischemic liver injury following different durations of ischemia[J]. Am J Physiol Gastrointest Liver Physiol, 2003, 284(3):G536–545.
[13] Sosna J, Philipp S, Fuchslocher Chico J, et al. Differences and Similarities in TRAIL- and Tumor Necrosis Factor-Mediated Necroptotic Signaling in Cancer Cells[J]. Mol Cell Biol, 2016,36(20): 2626–2644. doi: 10.1128/MCB.00941–15.
[14] Fu K, Pan H, Liu S, et al. Glycogen synthase kinase-3β regulates tumor necrosis factor-related apoptosis inducing ligand (TRAIL)-induced apoptosis via the NF-κB pathway in hepatocellular carcinoma[J]. Oncol Lett, 2015, 10(6): 3557–3564.
[15] Elmallah MI, Micheau O. Marine Drugs Regulating Apoptosis Induced by Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL)[J]. Mar Drugs, 2015, 13(11):6884–6909. doi:10.3390/md13116884.
[16] 梁艳, 杨再兴, 王皓, 等. TRAIL诱导正常人肝细胞线粒体损伤的实验研究[J]. 现代免疫学, 2009, 29(2):143–147.Liang Y, Yang ZX, Wang H, et al. Experimental study of TRAIL inducing mitochondria damage in normal liver cells[J]. Current Immunology, 2009, 29(2):143–147.
[17] Lee DH, Nam YJ, Kim YJ, et al. Rotundarpene prevents TRAIL-induced apoptosis in human keratinocytes by suppressing the caspase-8- and Bid-pathways and the mitochondrial pathway[J].Naunyn Schmiedebergs Arch Pharmacol, 2014, 387(12):1209–1219.doi: 10.1007/s00210–014–1051–8.
[18] 谭烨, 尤楠, 郑璐, 等. 过表达Tg737基因对肝癌细胞周期和凋亡的影响[J]. 中国普通外科杂志, 2017, 26(3):333–340. doi:10.3978/j.issn.1005–6947.2017.03.010.Tan Y, You N, Zheng L, et al. Influence of Tg737 gene overexpression on cell cycle and apoptosis of hepatocellular carcinoma cells[J]. Chinese Journal of General Surgery, 2017,26(3):333–340. doi:10.3978/j.issn.1005–6947.2017.03.010.
[19] 夏茜, 李晖, 吴杰, 等. 胃癌细胞中miR-455–3p的表达及其对增殖和凋亡的影响[J]. 中国普通外科杂志, 2017, 26(10):1272–1278.doi:10.3978/j.issn.1005–6947.2017.10.008.Xia Q, Li H, Wu J, et al.MiR-455–3p expression and its action on proliferation and apoptosis in gastric carcinoma cells [J]. Chinese Journal of General Surgery, 2017, 26(10):1272–1278. doi:10.3978/j.issn.1005–6947.2017.10.008.
[20] 张桂英, 彭杰, 肖志强, 等. 塞来昔布诱导大肠癌细胞凋亡与细胞色素C依赖性信号途径活化相关[J]. 中华消化杂志, 2004,24(9):537-540. doi:10.3760/j.issn:0254-1432.2004.09.008.Zhang GY, Peng J, Xiao ZQ, et al. Induction of apoptosis by celecoxib through activation of cytochrome C pathway in HT-29 cell[J]. Chinese Journal of Digestion, 2004, 24(9):537–540.doi:10.3760/j.issn:0254–1432.2004.09.008.
[21] 何青春, 刘婷, 周利平 等. 轻中度亚低温对大鼠心肺复苏后脑细胞caspase-3、Bcl-2和Bax蛋白表达的影响[J]. 南方医科大学学报, 2013, 33(10):1489–1493.He QC, Liu T, Zhou LP, et al. Effect of mild to moderate hypothermia on casepase-3, Bcl-2 and Bax expressions in brain tissue of rats after cardiopulmonary resuscitation[J]. Journal of Southern Medical University, 2013, 33(10):1489–1493.