直肠癌根治术中,准确的界定手术切除平面,清扫更多的淋巴结,减少周围脏器损伤尤其是盆腔自主神经损伤,是直肠癌标准根治手术的基本要求。腹腔镜手术相对于开腹手术来说,由于其放大视野作用,神经血管显示更清晰,损伤发生率更低[1]。但对于新辅助放化疗后的直肠癌手术患者,尤其是肥胖患者,由于组织水肿及纤维化,手术界面的判定困难,术中容易损伤盆腔自主神经,同时放化疗患者淋巴结检出数目减少,怎样减少盆腔神经损伤、检出更多微小淋巴结?笔者对96例新辅助放化疗后腹腔镜直肠癌根治术中分别采用术中经直肠上动脉内注射1%亚甲蓝8 mL及直接行根治术,并将两者进行对照研究,旨在探讨新辅助放化疗后腹腔镜直肠癌根治术中采用直肠上动脉亚甲蓝灌注对淋巴结检出数及男性术后性功能、排尿功能改变的影响。
1 资料与方法
1.1 一般资料
选择2013年3月—2017年4月期间在湘潭市中心医院胃肠外科和中山市陈星海医院普通外科收治的直肠癌患者96例临床资料,均为接受新辅助同步放化疗后进行了腹腔镜直肠癌根治术的男性患者,随机分为对照组48例和试验组48例。纳入及排除标准:⑴ 病理检查证实为距齿状线8 cm以内的腺癌;⑵ 经MRI证实为T3、T4或盆腔淋巴结肿大;⑶ 经CT等检查排除远处转移;⑷ 男性患者;⑸ 术前无性功能障碍及排尿功能障碍;⑹ 排除因急性梗阻、出血、穿孔行急诊手术。
1.2 新辅助放化疗
所有患者均接受术前新辅助同步放化疗。放疗总剂量45 Gy,5次/周,1.8 Gy/次,共5周。放疗期间同步服用卡培他滨(或希罗达)825 mg/m2,2次/d,连续5周。休息6~8周后手术治疗。
1.3 手术治疗
两组均按全直肠系膜切除(TME)原则进行保护自主神经的腹腔镜直肠癌根治术,包括腹会阴联合切除术(Miles术)或直肠前切除术(Dixon术)+末段回肠预防性造瘘术。两组患者均先游离暴露肠系膜下动脉以及左结肠动脉、乙状结肠动脉、直肠上动脉等分支,结扎肠系膜下静脉。试验组以持针器夹持5号头皮针经12 mm套管送入腹腔,并斜行15°穿刺直肠上动脉,见到回血后缓慢推入8 mL亚甲蓝,注射时间约2 min,见整个直肠及系膜染色后,沿染色界面进行后继手术。对照组则无亚甲蓝注射的操作步骤。两组均沿直肠系膜周围Toldt's间隙的无血管平面进行分离,在肠系膜下动脉后缘及骶岬前面保护好腹主动脉丛及腹下神经丛,并显露下腹下神经干(射精神经),在直肠前方Denonvilliers筋膜的分离中勿靠近精囊腺侧后方,以免损伤血管神经束,在直肠侧韧带分离过程中,勿靠近骨盆侧壁,以免损伤骨盆神经丛,导致勃起障碍。
1.4 观察指标
⑴ 淋巴结检获数:由手术医生进行淋巴结检获。对照组采用传统的触摸法。试验组打开系膜的浆膜面后,在黄色的脂肪背景下检出蓝色淋巴结,包括直径5 mm及以下的淋巴结。淋巴结和大体标本一同送常规病理检查、HE染色后,显微镜下判断是否有癌转移。⑵ 术后1年的射精功能:射精功能分为3级,I级为射精功能基本正常,射精量略减少或正常;II级为存在射精功能障碍,伴有逆行射精等现象;III级完全无射精[2]。⑶ 术后1年的勃起功能:勃起功能分为3级,I级为勃起功能基本正常;II级为部分勃起且勃起硬度降低;III级丧失勃起功能,无法勃起[2]。⑷ 术后排尿功能:术后排尿功能按照SAITO分级标准[3]分为4级(I级:患者术后能正常排尿,无排尿功能障碍;II级:患者术后表现尿频,残余尿量少于50 mL,存在轻度排尿功能障碍;III级:患者术后残余尿量超过50 mL,但很少需要进行导尿治疗,排尿障碍中度;IV级:患者术后存在尿潴留或尿失禁等症状,需要进行导尿治疗,排尿障碍表现为重度)。
1.5 统计学处理
比较试验组和对照组的差异,其中定量资料(年龄、肿瘤直径、肿瘤距肛门距离、检出淋巴结个数)先进行Kolmogorov-Smirnov正态性检验,符合正态分布者采用两独立样本t检验,不符合者采用两独立样本Wilcoxon秩和检验;定性资料(手术方式)比较采用χ2检验,不满足χ2检验条件者用Fisher精确概率计算;等级资料(肿瘤病理类型、肿瘤分期、术后1年射精功能、术后1年勃起功能、术后排尿功能)采用两独立样本Wilcoxon秩和检验。使用SPSS 13.0统计软件,按α=0.05检验水准,P<0.05为差异有统计学意义。
2 结 果
2.1 两组患者的基线资料
将对照组与试验组基线资料进行比较,结果显示,两组患者间的年龄、病理类型、肿瘤分期、肿瘤大小及距肝门距离差异均无统计学意义(均P>0.05)(表1)。
表1 两组患者基线资料(n=48)
Table 1 The baseline data of the two groups of patients (n=48)

?
2.2 两组患者的观察指标比较
两组间手术方式无统计学差异(P=0.408);试验组淋巴结清扫数目多于对照组[(15.04±4.063)枚 vs.(12.23±2.991)枚,P<0.05];术后1年试验组射精功能I、II、III级者分别为41(85.4%)、7(14.6%)、0(0)例,对照组为31(64.6%)、13(27.1%)、4(8.3%)例,试验组射精功能优于对照组(P=0.014);术后1年试验组患者勃起功能I、II、III级者分别为42(87.5%)、6(12.5%)、0(0)例,对照组为32(66.7%)、15(31.3%)、1(2.1%)例,试验组勃起功能明显优于对照组(P=0.015);试验组术后排尿功能I、II、III、IV级者分别为35(72.9%)、12(25.0%)、1(2.1%)、0(0)例,对照组为26(54.2%)、13(27.1%)、5(10.4%)、4(8.3%)例,试验组术后排尿明显优于对照组(P=0.045)(表2)。
表2 两组患者术中术后情况比较(n=48)
Table 2 Comparison if the intra- and postoperative variables of the two groups of patients (n=48)

资料 对照组 试验组 t/χ2/Z P手术方式[n(%)]Dixon+回肠造瘘 26(54.2) 30(62.5) 0.6860.408 Miles 22(45.8) 18(37.5)检出淋巴结(枚,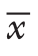 ±s) 12.23±2.99115.04±4.063-3.862 <0.05术后1年射精功能[n(%)]I 31(64.6) 41(85.4)II 13(27.1) 7(14.6) -2.4670.014 III 4(8.3) 0(0)术后1年勃起功能[n(%)]I 32(66.7) 42(87.5)II 15(31.3) 6(12.5) -2.4420.015 III 1(2.1) 0(0)术后排尿功能[n(%)]I 26(54.2) 35(72.9)II 13(27.1) 12(25.0) 8.035 0.045 III 5(10.4) 1(2.1)IV 4(8.3) 0(0)
±s) 12.23±2.99115.04±4.063-3.862 <0.05术后1年射精功能[n(%)]I 31(64.6) 41(85.4)II 13(27.1) 7(14.6) -2.4670.014 III 4(8.3) 0(0)术后1年勃起功能[n(%)]I 32(66.7) 42(87.5)II 15(31.3) 6(12.5) -2.4420.015 III 1(2.1) 0(0)术后排尿功能[n(%)]I 26(54.2) 35(72.9)II 13(27.1) 12(25.0) 8.035 0.045 III 5(10.4) 1(2.1)IV 4(8.3) 0(0)
3 讨 论
3.1 亚甲蓝灌注对于手术操作及术后并发症的影响
近年来随着技术及器械的进步,在有条件的医院,腹腔镜手术已经成为直肠癌患者的首选术式[4]。同时,对于中晚期直肠癌,术前新辅助放化疗也已成为标准治疗规范[5-6]。而对于接受过术前新辅助放化疗的患者,由于放疗导致局部组织的水肿及纤维化,使手术区缺乏明确界线,难以区分包裹在盆筋膜脏层内的直肠系膜和盆筋膜脏层外的周围组织。腹腔镜下如果手术操作不当、手术平面偏差可能引起盆腔内脏神经损伤,导致性功能障碍、排尿功能障碍。
亚甲蓝是临床上常用的染色剂,广泛应用于普外科各种手术之中,作用包括前哨淋巴结检测、肝叶肝段精确定位等[7-10]。而在结直肠癌患者中,亚甲蓝也多应用于染色内镜检查、内镜下定位、吻合测漏试验、淋巴结检出等多个方面[11-15]。直肠癌手术中经直肠上动脉注射亚甲蓝后,由于盆筋膜的分隔作用,盆筋膜脏层内的直肠系膜染成蓝色,而盆筋膜脏层外的周围组织如盆筋膜壁层及下方的自主神经不染色,因此为切除直肠系膜提供了清晰的手术界面,有利于术中分离操作的实施。贺孝文等[16]的一项研究显示在直肠癌根治手术中,亚甲蓝动脉灌注能够减少盆腔自主神经损伤,从而改善了术后排尿功能及性功能。但该研究主要关注开腹手术的情况,且未涉及新辅助放化疗。本研究则进一步将样本限制在新辅助放化疗后的腹腔镜直肠癌根治手术患者中,结果同样显示术后试验组男性性功能、排尿功能优于对照组,差异有统计学意义。
3.2 亚甲蓝灌注对于淋巴结检出的影响
直肠癌术前新辅助放化疗能提高肿瘤切除率和保肛率,可能降低肿瘤分期,改善患者生存率,但放疗亦可导致组织纤维化、淋巴结退缩,使术后肿瘤标本中淋巴结检出困难、检出数目减少,可能影响分期准确性。本研究中对照组淋巴结检出数目仅(12.23±2.991)枚,其中有高达20例(41%)未满足NCCN指南要求的12枚标准,由此可见一斑。多篇文献[17-22]显示,对于直肠癌术后离体标本,亚甲蓝动脉注射染色可增加淋巴结检出数目,有助于提高分期准确性,在新辅助放疗后人群中尤为重要[23-24]。但以上这些研究均将亚甲蓝注射应用在术后离体标本中,并未研究手术过程中亚甲蓝动脉注射的作用。国内亦有研究在手术过程中进行体内亚甲蓝动脉注射染色,发现同样有助于提高标本淋巴结检出率[25],但并未关注新辅助放疗后的情况,亦未关注腹腔镜下手术的情况。在本研究中则重点观察了新辅助同步放化疗后行腹腔镜手术的人群,发现腹腔镜下直肠上动脉注射亚甲蓝能显著提高淋巴结检出率[(15.04±4.063)枚vs.(12.23±2.991)枚,P<0.05]。但试验组中仍有6例达不到12枚的标准,这估计同样与放化疗后淋巴结纤维化、直径变小有关。
3.3 腹腔镜术中直肠上动脉亚甲蓝灌注的操作要点
腹腔镜下准确的进行直肠上动脉穿刺注射亚甲蓝是本研究中的技术操作重点及难点。与开腹手术及离体标本相比,腹腔镜下血管穿刺存在一定难度,角度及力度掌握不好则很容易穿破血管导致出血或将亚甲蓝注射到血管以外,导致术野大片蓝染影响后继操作;反复穿刺未成功则可能导致穿刺针或血管内血凝块堵塞,影响继续穿刺注射。笔者的经验是,首先应该充分游离暴露肠系膜下动脉及左结肠动脉、乙状结肠动脉、直肠上动脉各分支,推荐行鞘内游离,既有利于淋巴结的清扫,也有利于下一步的直肠上动脉穿刺,因为如果保留血管鞘,则血管外组织过厚可增大穿刺难度,也可能穿入血管鞘与血管壁之间的间隙导致假染色。此外,建议选择5号头皮针,经12 mm套管送入腹腔内,输液管留置腹腔外,预先连接装有肝素盐水的注射器,助手将乙状结肠系膜充分展开并绷直裸化的直肠上动脉,术者右手以持针器夹持针头,左手持钳轻夹连接针头的输液管以调节角度,斜行15°缓慢穿刺入直肠上动脉,回抽见到回血后可先推注少量肝素盐水抗凝,然后再缓慢推入8 mL亚甲蓝进行染色。
本研究中重点关注了新辅助放化疗导致局部组织水肿纤维化及淋巴结退缩的情况下腹腔镜直肠上动脉亚甲蓝灌注的作用,与既往研究相比有一定创新性,初步显示亚甲蓝灌注能使手术层面更清晰、减少盆腔内脏神经损伤、提高淋巴结检出率。诚然,由于研究时间及样本量的限制,本课题仍只是术后短期效果的初步研究与探讨,并未就患者长期生存率及肿瘤复发率等进行探讨。下一步有望在后继研究中扩大样本量,进一步验证以上相关结论,并在长期随访观察中继续研究其在患者生存率及肿瘤复发率方面的意义。
综上所述,对于新辅助放化疗后的患者,直肠上动脉亚甲蓝灌注有助于在腹腔镜直肠癌根治术中保护盆腔内脏神经,有效减少术后性功能损伤与排尿功能障碍,并提高淋巴结清扫率及术后淋巴结检出率,值得在直肠癌手术中推广应用。
[1]池畔, 陈致奋.腹腔镜直肠全系膜切除术中的泌尿及性功能保护[J].外科理论与实践, 2012, 17(3):220–223.doi:10.3969/j.issn.1007–1096.2012.03.006.Chi P, Chen ZF.Protection of urinary function and sexual function in laparoscopic total mesorectal excision[J].Journal of Surgery Concepts & Practice, 2012, 17(3):220–223.doi:10.3969/j.issn.1007–1096.2012.03.006.
[2]李锡丁, 杜旭东, 陈力平.保留盆腔自主神经在全直肠系膜切除术中的应用研究[J].重庆医学, 2012, 41(21):2166–2167.doi:10.3969/j.issn.1671–8348.2012.21.018.Li XD, Du XD, Chen LP.The effects of pelvic autonomic nerve preservation in total mesorectal excision[J].Chongqing Medicine, 2012, 41(21):2166–2167.doi:10.3969/j.issn.1671–8348.2012.21.018.
[3]Saito N, Sarashina H, Nunomura M, et al.Clinical evaluation of nerve-sparing surgery combined with preoperative radiotherapy in advanced rectal cancer patients[J].Am J Surg, 1998, 175(4):277–282.
[4]熊懿.腹腔镜直肠全系膜切除术治疗中、低位直肠癌的临床疗效分析[J].中国普通外科杂志, 2015, 24(4):616–618.doi:10.3978/j.issn.1005–6947.2015.04.030.Xiong Y.Laparoscopic total mesorectal excision for middle and low level rectal cancer: analysis of clinical outcomes[J].Chinese Journal of General Surgery, 2015, 24(4):616–618.doi:10.3978/j.issn.1005–6947.2015.04.030.
[5]冯鹏才, 杨金煜, 唐明杰, 等.进展期中低位直肠癌新辅助放化疗后肿瘤部位及手术方式对患者预后的影响[J].中国普通外科杂志, 2015, 24(6):895–898.doi:10.3978/j.issn.1005–6947.2015.06.027.Feng PC, Yang JY, Tang MJ, et al.Influence of the tumor site and surgical methods on prognosis of patients with the advanced middle and low rectal cancer following neoadjuvant chemoradiation[J].Chinese Journal of General Surgery, 2015, 24(6):895–898.doi:10.3978/j.issn.1005–6947.2015.06.027.
[6]曹金鹏, 彭翔, 李国新, 等.新辅助治疗后腹腔镜直肠癌根治术的中短期疗效[J].中国普通外科杂志, 2014, 23(4):442–446.doi:10.7659/j.issn.1005–6947.2014.04.008.Cao JP, Peng X, Li GX, et al.Short-and medium-term outcomes of laparoscopic radical resection for rectal cancer after neoadjuvant therapy[J].Chinese Journal of General Surgery, 2014, 23(4):442–446.doi:10.7659/j.issn.1005–6947.2014.04.008.
[7]刘宏斌, 韩晓鹏, 孟文喆, 等.胃癌前哨淋巴结临床意义的研究[J].中国普通外科杂志, 2006, 15(2):81–84.doi:10.3969/j.issn.1005–6947.2006.02.001.Liu HB, Han XP, Meng WZ, et al.Study on the clinical significance of sentinel nodes in gastric cancer[J].Chinese Journal of General Surgery, 2006, 15(2):81–84.doi:10.3969/j.issn.1005–6947.2006.02.001.
[8]张贾震男, 欧江华, 张晨光, 等.联合吲哚菁绿荧光法与蓝染法行乳腺癌前哨淋巴结活检的临床研究[J].中国普通外科杂志,2016, 25(5):705–710.doi:10.3978/j.issn.1005–6947.2016.05.014.Zhang JZN, Ou JH, Zhang CG, et al.Combined tracing method of indocyanine green fluorescence and methylene blue dyeing in sentinel lymph node biopsy of breast cancer[J].Chinese Journal of General Surgery, 2016, 25(5):705–710.doi:10.3978/j.issn.1005–6947.2016.05.014.
[9]陈晓亮, 王川红, 宋志, 等.肝血管成像三维重建联合区域血流阻断美蓝持久染色在精准肝切除手术中的应用[J].中国普通外科杂志, 2015, 24(7):1001–1006.doi:10.3978/j.issn.1005–6947.2015.07.015.Chen XL, Wang CH, Song Z, et al.Application of hepatic angiography with 3-D reconstruction plus regional blood flow occlusion and persistent methylene blue dyeing in precise hepatectomy[J].Chinese Journal of General Surgery, 2015,24(7):1001–1006.doi:10.3978/j.issn.1005–6947.2015.07.015.
[10]吴殿文, 王锡山.结直肠癌前哨淋巴结活检的临床意义[J].中华结直肠疾病电子杂志, 2014, 3(4):54–56.doi:10.3877/cma.j.issn.2095–3224.2014.04.15.Wu DW, Wang XS.Clinical value of SLN in surgery on colorectal cancer [J].Chinese Journal of Colorectal Diseases, 2014, 3(4):54–56.doi:10.3877/cma.j.issn.2095–3224.2014.04.15.
[11]李会霞, 王再见, 江海燕, 等.不同浓度亚甲蓝染色对结直肠病变EMR及ESD的影响[J].中华腔镜外科杂志:电子版, 2015,8(3):34–37.doi:10.3877/cma.j.issn.1674–6899.2015.03.009.Li HX, Wang ZJ, Jiang HY, et al.Different concentrations of methylene blue staining of EMR and ESD in the colorectal operation[J].Chinese Journal of Laparoscopic Surgery:Electronic Edition, 2015, 8(3):34–37.doi:10.3877/cma.j.issn.1674–6899.2015.03.009.
[12]王新团, 贾蓬勃, 王华.内镜无法切除的结直肠息肉行结肠镜钛夹定位与美蓝定位的方案应用分析[J].腹腔镜外科杂志, 2015,20(3):167–170.doi:10.13499/j.cnki.fqjwkzz.2015.03.167.Wang XT, Jia PB, Wang H.The study of the efficacies of preoperative location with titanium clip and methylene blue staining in patients with endoscopic failure treatment of colorectal polyps[J].Journal of Laparoscopic Surgery, 2015, 20(3):167–170.doi:10.13499/j.cnki.fqjwkzz.2015.03.167.
[13]陈玉祥, 黄卫, 李俊, 等.直肠充气试验+美蓝试验在直肠癌TME术中的应用研究[J].中国普外基础与临床杂志, 2013,20(9):1034–1037.doi: 10.7507/1007–9424.20130258.Chen YX, Huang W, Li J, et al.The Application of Air Leak Test Combined with Methylene Blue Solution Leak Test in Detection of Anastomotic Leakageafter Total Mesorectal Excision in Rectal Cancer[J].Chinese Journal of Bases and Clinics In General Surgery,2013, 20(9):1034–1037.doi: 10.7507/1007–9424.20130258.
[14]陈玉祥, 黄国飞, 李俊, 等.亚甲蓝在结直肠吻合术中的应用[J].四川医学, 2013, 34(7):1038–1039.doi:10.3969/j.issn.1004–0501.2013.07.065.Chen YX, Huang GF, Li J, et al.Application of methylene blue in colorectal anastomosis[J].Sichuan Medical Journal, 2013,34(7):1038–1039.doi:10.3969/j.issn.1004–0501.2013.07.065.
[15]毛方术, 顾晓辉, 字灿忠, 等.直肠上动脉亚甲蓝灌注法增加直肠癌淋巴结获检数[J].西南国防医药, 2013, 23(5):482–483.doi:10.3969/j.issn.1004–0188.2013.05.006.Mao FS, Gu XH, Zi CZ, et al.Perfusion with methylene blue via superior rectal artery can increase detected number of lymph node in rectal cancer specimen[J].Medical Journal of National Defending Forces in Southwest China, 2013, 23(5):482–483.doi:10.3969/j.issn.1004–0188.2013.05.006.
[16]贺孝文, 李广权, 张锐江, 等.亚甲蓝动脉灌注应用于直肠癌全直肠系膜切除术对男性患者术后排尿功能及性功能的影响[J].中华胃肠外科杂志, 2016, 19(4):414–417.doi:10.3760/cma.j.issn.1671–0274.2016.04.014.He XW, Li GQ, Zhang RJ, et al.Effect of arterial infusion with methylene blue during total mesorectal excision on urination function and sexual function in male patients with rectal cancer[J].Chinese Journal of Gastrointestinal Surgery, 2016, 19(4):414–417.doi:10.3760/cma.j.issn.1671–0274.2016.04.014.
[17]Vasala A, Nair HG, Rao ST, et al.Impact of methylene blue staining in the retrieval of lymph nodes in resected colorectal cancer specimens[J].Indian J Pathol Microbiol, 2016, 59(4):504–506.doi:10.4103/0377–4929.191804.
[18]Reima H, Saar H, Innos K, et al.Methylene blue intra-arterial staining of resected colorectal cancer specimens improves accuracy of nodal staging: A randomized controlled trial[J].Eur J Surg Oncol, 2016, 42(11):1642–1646.doi: 10.1016/j.ejso.2016.06.001.
[19]Kir G, Alimoglu O, Sarbay BC, et al.Ex vivo intra-arterial methylene blue injection in the operation theater may improve the detection of lymph node metastases in colorectal cancer[J].Pathol Res Pract, 2014, 210(12):818–821.doi: 10.1016/j.prp.2014.09.003.
[20]Borowski DW, Banky B, Banerjee AK, et al.Intra-arterial methylene blue injection into ex vivo colorectal cancer specimens improves lymph node staging accuracy: a randomized controlled trial[J].Colorectal Dis, 2014, 16(9):681–689.doi: 10.1111/codi.12681.
[21]Liu J, Huang P, Zheng Z, et al.Modified methylene blue injection improves lymph node harvest in rectal cancer[J].ANZ J Surg, 2017,87(4):247–251.doi: 10.1111/ans.12889.
[22]陈涛, 华一兵.纳米碳和亚甲蓝在结直肠癌根治术淋巴结检出中的应用[J].临床和实验医学杂志, 2016, 15(24):2410–2414.doi:10.3969/j.issn.1671–4695.2016.24.009.Chen T, Hua YB.Application of lymph node labeling in colorectal cancer surgery[J].Journal of Clinical and Experimental Medicine, 2016, 15(24):2410–2414.doi:10.3969/j.issn.1671–4695.2016.24.009.
[23]Münster M, Hanisch U, Tuffaha M, et al.Ex Vivo Intra-arterial Methylene Blue Injection in Rectal Cancer Specimens Increases the Lymph-Node Harvest, Especially After Preoperative Radiation[J].World J Surg, 2016, 40(2):463–470.doi: 10.1007/s00268–015–3230–2.
[24]刘健培, 黄品婕, 黄江龙, 等.经肠系膜下动脉注射美蓝对新辅助化疗后直肠癌的淋巴结检出的影响[J].中华医学杂志, 2015,95(22):1736–1738.doi:10.3760/cma.j.issn.0376–2491.2015.22.007.Liu JP, Huang PJ, Huang JL, et al.Injection of methylene blue into inferior mesenteric artery improves lymph node harvest in rectal cancer after neoadjuvant chemotherapy[J].National Medical Journal of China, 2015, 95(22):1736–1738.doi:10.3760/cma.j.issn.0376–2491.2015.22.007.
[25]向威, 符艳艳, 徐长青, 等.美兰直肠上动脉注射显影在直肠癌根治术中的应用价值[J].河南中医, 2014, 34(6):270–271.Xiang W, Fu YY, Xu CQ, et al.Meilan rectal artery injection on developing application value in colorectal cancer radical prostatectomy[J].Henan Traditional Chinese Medicine, 2014,34(6):270–271.
