Stanford B型主动脉夹层是一种极其凶险的心血管疾病,对于患者有生命危害大、起病快的特点,Stanford B型夹层患者病死率虽然较A型低,但仍有10%患者在发病的30 d内死亡,更为严重的是对于该疾病的高危患者,其病死率将近70%[1]。相关研究[2]表明,Stanford B型主动脉夹层发病率呈增长趋势。近年来,胸主动脉腔内修复术(thoracic endovascular aortic repair,TEVAR)因其微创、术后恢复快等优点,已经成为Stanford B型主动脉夹层的主要手术治疗方式。目前对于Stanford B型主动脉夹层研究重点在于T E V A R手术的条件、破口的处理等方面,对于T E V A R术后急性肾功能损害方面报道相对较少。TEVAR术后急性肾损伤的发生在多项研究中,发生率从1.5%~34%不等[3-5]。本研究旨通过分析Stanford B型主动脉夹层术后出现急性肾损伤的危险因素,对可能出现急性肾损伤高危患者的早期预防提供参考。
1 资料与方法
1.1 研究对象
2013—2016年,安徽省立医院收治Stanford B型主动脉夹层患者共241例,根据排除标准共纳入193例患者。入选标准:(1)根据CTA结果明确诊断为Stanford B型主动脉夹层的患者;(2)入院后成功行TEVAR术患者。排除标准:(1)既往有主动脉病变行腔内修复术的患者;(2)被认为在技术上不成功的患者;(3)合并结缔组织疾病的患者;(4)接受其他肾动脉血流重建的患者;(5)术后缺乏肾功能检查或缺乏CTA影像学资料的患者。
1.2 研究方法
收集患者一般资料(性别、年龄、既往史、个人史等)、入院时心率、收缩压、舒张压、白细胞计数、术前及术后48 h内血清肌酐(Scr)水平、术后48 h内最高体温、手术时间、机械通气时间、术中造影剂用量、是否急性期手术、平均住院日及入院胸腹主动脉CTA明确肾动脉受累情况,根据急性肾损伤网(the Acute Kidney Injury Network,AKIN)标准将急性肾损伤定义为:在48 h内Scr上升≥26.4 μmol/L或Scr上升至≥基础值的1.5倍[6],为急性肾损伤。
1.3 术后随访
患者出院后随访采用电话和门诊复诊等方式。随访时间为术后1、6个月与1年,以后1次/年。随访内容主要包括患者血压控制情况、肾功能情况及生存情况(死亡患者需明确其死亡时间及死亡原因)。
1.4 统计学处理
数据分析采用SPSS 19.0统计软件进行分析,连续变量采用均数±标准差( ±s)或中位数(四分位数间距)[M(Q)]表示,组间差异采用t检验或非参数Mann-whitney U检验。计数资料采用频数和百分比[n(%)]表示,组间差异采用χ2检验或Fisher精确检验。将单因素分析有统计学意义的变量引入多因素Logistic回归分析模型,生存分析采用Cox回归分析,P<0.05为差异有统计学意义。
±s)或中位数(四分位数间距)[M(Q)]表示,组间差异采用t检验或非参数Mann-whitney U检验。计数资料采用频数和百分比[n(%)]表示,组间差异采用χ2检验或Fisher精确检验。将单因素分析有统计学意义的变量引入多因素Logistic回归分析模型,生存分析采用Cox回归分析,P<0.05为差异有统计学意义。
2 结 果
2.1 术前一般资料与术中情况
符合标准的193患者例中,男157例,女36例;合并高血压138例(71.50%)、糖尿病16例(8.30%)、吸烟史65例(33.68%)。36例(18.7%)发生急性肾损伤,急性肾损伤组糖尿病病史比例明显高于非急性肾损伤组(16.7% vs.6.37%,P=0.043)、急性肾损伤组的收缩压、舒张压均高于非急性肾损伤组(均P<0.01)。两组之间手术时间、机械通气时间、是否急性期手术、平均住院日差异无统计学意义(均P>0.05)。术中造影剂用量,急性肾损伤组明显高于非急性肾损伤组(P<0.01)(表1)。
表1 术后急性肾损伤组与非急性肾损伤组一般资料和手术资料单因素分析结果
Table 1 Results of univariate analysis of the general data and surgical variables of patients with and without acute kidney injury
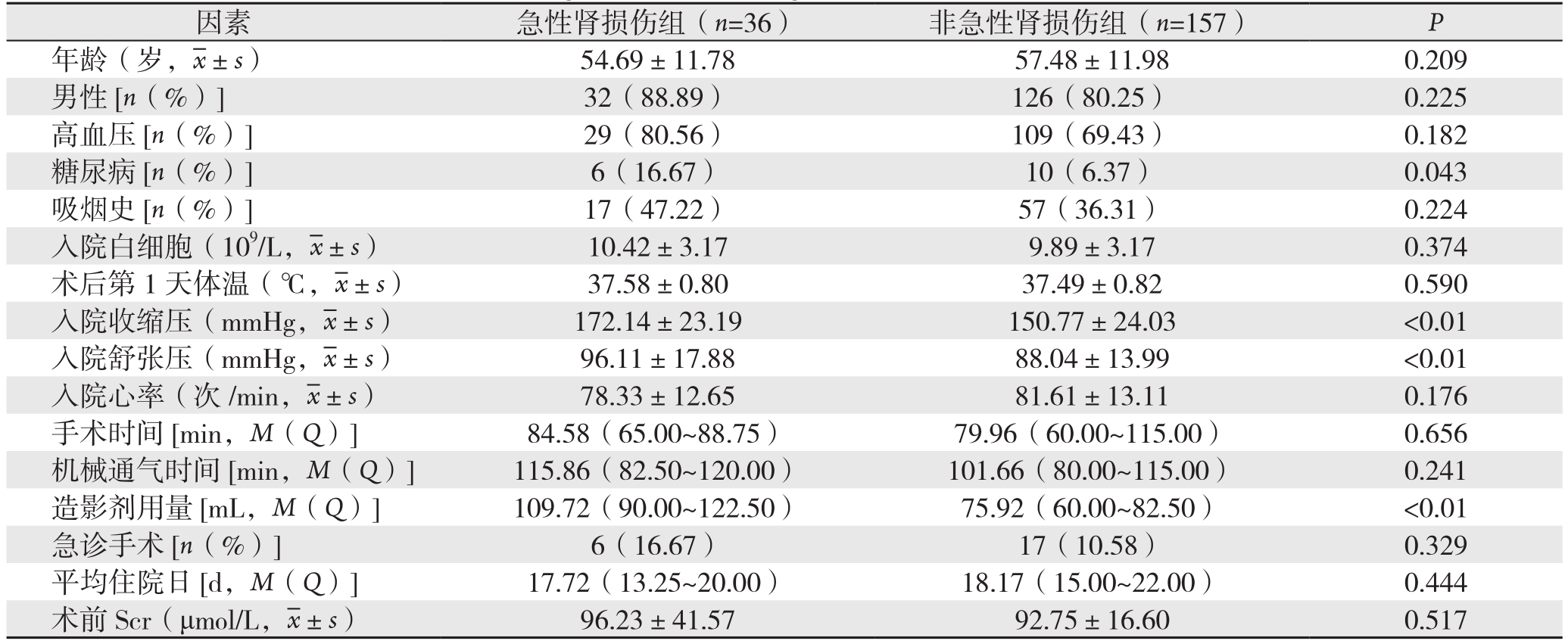
因素 急性肾损伤组(n=36)非急性肾损伤组(n=157)P年龄(岁, ±s)54.69±11.7857.48±11.980.209男性[n(%)] 32(88.89)126(80.25)0.225高血压[n(%)] 29(80.56)109(69.43)0.182糖尿病[n(%)] 6(16.67)10(6.37)0.043吸烟史[n(%)] 17(47.22)57(36.31)0.224入院白细胞(109/L,
±s)54.69±11.7857.48±11.980.209男性[n(%)] 32(88.89)126(80.25)0.225高血压[n(%)] 29(80.56)109(69.43)0.182糖尿病[n(%)] 6(16.67)10(6.37)0.043吸烟史[n(%)] 17(47.22)57(36.31)0.224入院白细胞(109/L, ±s)10.42±3.179.89±3.170.374术后第1天体温(℃,
±s)10.42±3.179.89±3.170.374术后第1天体温(℃, ±s)37.58±0.8037.49±0.820.590入院收缩压(mmHg,
±s)37.58±0.8037.49±0.820.590入院收缩压(mmHg, ±s)172.14±23.19150.77±24.03 <0.01入院舒张压(mmHg,
±s)172.14±23.19150.77±24.03 <0.01入院舒张压(mmHg, ±s)96.11±17.8888.04±13.99 <0.01入院心率(次/min,
±s)96.11±17.8888.04±13.99 <0.01入院心率(次/min, ±s)78.33±12.6581.61±13.110.176手术时间[min,M(Q)] 84.58(65.00~88.75)79.96(60.00~115.00)0.656机械通气时间[min,M(Q)] 115.86(82.50~120.00)101.66(80.00~115.00)0.241造影剂用量[mL,M(Q)] 109.72(90.00~122.50)75.92(60.00~82.50)<0.01急诊手术[n(%)] 6(16.67)17(10.58)0.329平均住院日[d,M(Q)] 17.72(13.25~20.00)18.17(15.00~22.00)0.444术前 Scr(μmol/L,
±s)78.33±12.6581.61±13.110.176手术时间[min,M(Q)] 84.58(65.00~88.75)79.96(60.00~115.00)0.656机械通气时间[min,M(Q)] 115.86(82.50~120.00)101.66(80.00~115.00)0.241造影剂用量[mL,M(Q)] 109.72(90.00~122.50)75.92(60.00~82.50)<0.01急诊手术[n(%)] 6(16.67)17(10.58)0.329平均住院日[d,M(Q)] 17.72(13.25~20.00)18.17(15.00~22.00)0.444术前 Scr(μmol/L, ±s)96.23±41.5792.75±16.600.517
±s)96.23±41.5792.75±16.600.517
2.2 主动脉夹层累及特点
根据CTA结果,肾动脉血供包括真腔、假腔、真假腔3种形式,肾脏的血供方式,急性肾损伤组与非急性肾损伤组无统计学差异(P左肾动脉=0.071、P右肾动脉=0.123)。但肾动脉受累数量两组间存在统计学差异,双侧肾动脉均受累出现急性肾损伤明显高于单侧肾动脉受累及肾动脉未受累患者(P=0.013)(表2)。
2.3 危险因素回归分析
单因素分析中有统计学意义的因素引入多因素Logistic回归分析中显示:糖尿病病史(OR=4.458,95% CI=1.176~16.897,P=0.028)、入院收缩压(OR=1.036,95% CI=1.011~1.063,P<0.01)、造影剂用量(OR=1.025,95% CI=1.012~1.038,P<0.01)、双侧肾动脉受累(OR=3.130,95% CI= 1.222~8.017,P=0.017)是TEVAR术后出现急性肾损伤的独立危险因素(表3)。
表2 急性肾损伤组与非急性肾损伤组肾动脉受累情况分析[n(%)]
Table 2 Analysis of the involvement of the renal arteries in patients with and without acute kidney injury [n(%)]
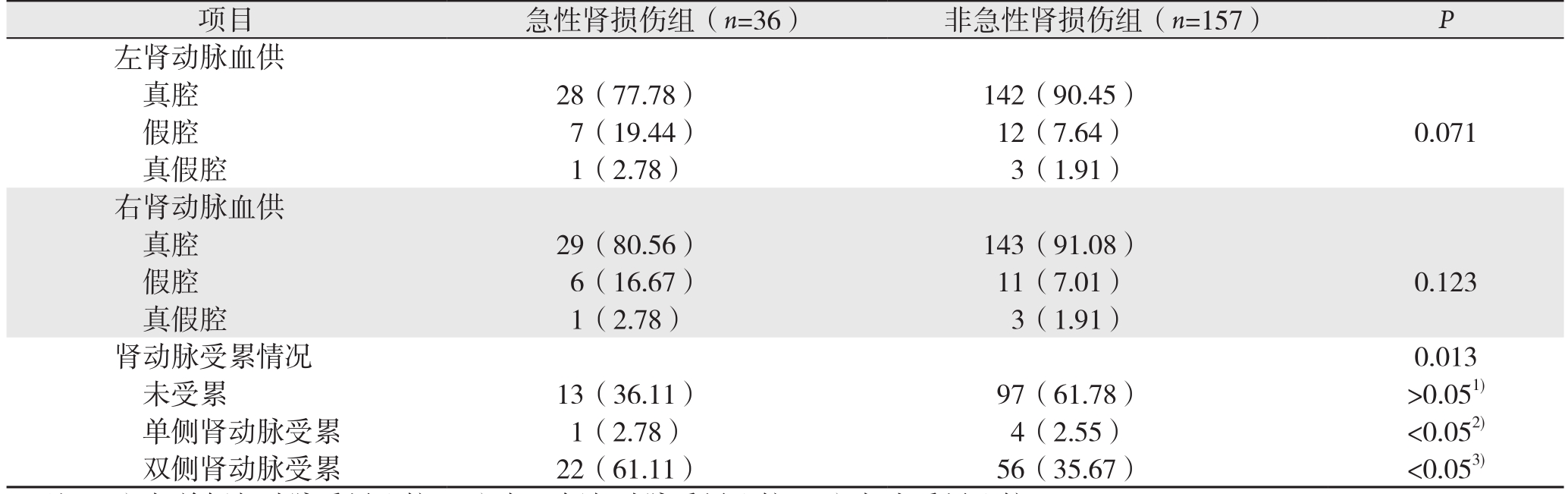
注:1)与单侧肾动脉受累比较;2)与双侧肾动脉受累比较;3)与未受累比较
Note:1)Compared with unilateral renal artery involvement; 2)Compared with bilateral renal artery involvement; 3)Compared with no renal artery involvement
项目 急性肾损伤组(n=36)非急性肾损伤组(n=157)P左肾动脉血供真腔 28(77.78)142(90.45)假腔 7(19.44)12(7.64)0.071真假腔 1(2.78)3(1.91)右肾动脉血供真腔 29(80.56)143(91.08)假腔 6(16.67)11(7.01)0.123真假腔 1(2.78)3(1.91)肾动脉受累情况0.013未受累 13(36.11)97(61.78)>0.051)单侧肾动脉受累 1(2.78)4(2.55)<0.052)双侧肾动脉受累 22(61.11)56(35.67)<0.053)
表3 TEVAR术后出现急性肾损伤的独立危险因素Logistic分析
Table 3 Logistic analysis of the risk factors for acute kidney injury after TEVAR
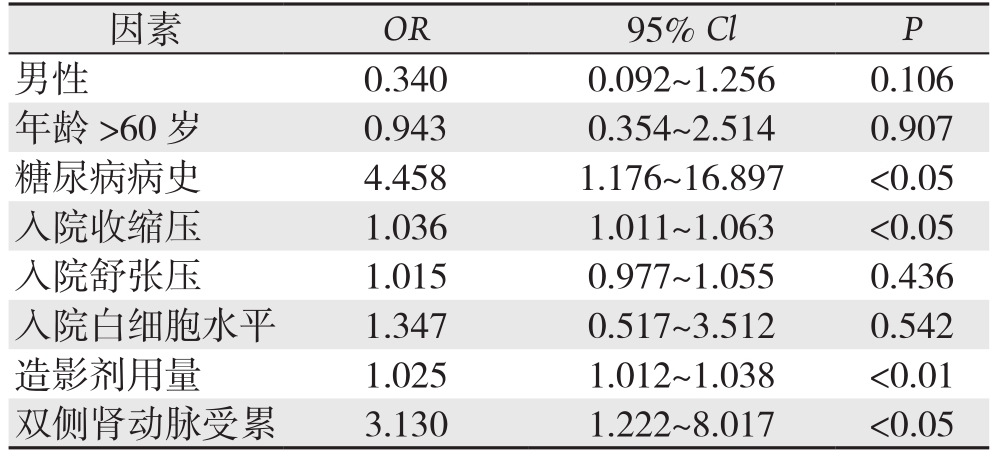
因素 OR 95% Cl P男性 0.3400.092~1.2560.106年龄 >60岁 0.9430.354~2.5140.907糖尿病病史 4.4581.176~16.897 <0.05入院收缩压 1.0361.011~1.063 <0.05入院舒张压 1.0150.977~1.0550.436入院白细胞水平 1.3470.517~3.5120.542造影剂用量 1.0251.012~1.038 <0.01双侧肾动脉受累 3.1301.222~8.017 <0.05
2.4 随访结果
急性肾损伤组随访人数31例,失访5例。非急性肾损伤组随访人数142例,失访11例。平均随访时间18.3个月。术后30 d随访结果,急性肾损伤组死亡患者5例,其中3例死于脑血管疾病,1例死于急性心肌梗死,1例死于重度肺部感染;非急性肾损伤组死亡患者6例,其中2例脑血管意外死亡,3例夹层破裂死亡,1例患者多脏器功能衰竭死亡。术后18个月随访结果,急性肾损伤组新增死亡患者2例,其中1例夹层破裂死亡,1例不明原因死亡;非急性肾损伤组新增死亡患者3例,1例死于脑血管意外,1例死于恶性肿瘤,1例死于呼吸衰竭,1例死于二次手术。两组总体病死率有统计学差异(22.6% vs. 6.3%,χ2=8.00,P<0.005)。TEVAR术后患者早期死亡单因素分析,糖尿病病史同时也是患者术后死亡的影响因素(表4)。随访18个月内死亡患者中,通过Cox回归分析显示,术前糖尿病会增加TEVAR术后早期死亡风险(OR=20.8,P=0.03),患者的生存曲线见图1。
表4 TEVAR术后患者早期死亡风险单因素分析结果
Table 4 Results of univariate analysis of risk factors for early death in patients after TEVAR

因素 死亡(n=16)存活(n=157)P年龄(岁, ±s)60.69±12.8756.69±11.850.204高血压[n(%)] 13(81.25)115(73.25)0.487糖尿病[n(%)] 6(37.50)2(1.27)<0.01吸烟史[n(%)] 7(43.75)68(43.31)0.973入院白细胞(109/L,
±s)60.69±12.8756.69±11.850.204高血压[n(%)] 13(81.25)115(73.25)0.487糖尿病[n(%)] 6(37.50)2(1.27)<0.01吸烟史[n(%)] 7(43.75)68(43.31)0.973入院白细胞(109/L, ±s)9.47±3.049.96±3.050.520入院收缩压(mmHg,
±s)9.47±3.049.96±3.050.520入院收缩压(mmHg, ±s)163.13±20.44152.25±25.870.105入院舒张压(mmHg,
±s)163.13±20.44152.25±25.870.105入院舒张压(mmHg, ±s)95.63±18.9188.52±14.170.066入院心率(次/min,
±s)95.63±18.9188.52±14.170.066入院心率(次/min, ±s)78.50±10.5581.80±13.300.337造影剂用量[mL,M(Q)] 88.44(52.50~100.00)81.24(60.00~90.00)0.329双侧肾动脉受累[n(%)] 7(43.75)47(29.94)0.256
±s)78.50±10.5581.80±13.300.337造影剂用量[mL,M(Q)] 88.44(52.50~100.00)81.24(60.00~90.00)0.329双侧肾动脉受累[n(%)] 7(43.75)47(29.94)0.256

图1 是否合并糖尿病病史患者的生存时间曲线
Figure 1 Survival curves of patients with and without a history of diabetes
3 讨 论
Stanford B型主动脉夹层以往行开放手术治疗,其手术难度大、术后并发症多,围手术期死亡风险极高。随着介入医疗技术发展,腔内治疗具有的微创、安全、有效的优点得到了越来越广泛的认可,目前已经成为Stanford B型主动脉夹层治疗的首选方式[7-8]。TEVAR术后出现急性肾损伤是较为常见的术后并发症,同时也是Stanford B型主动脉夹层术后30 d及1年死亡的独立危险因素[9]。目前对于Stanford B型主动脉夹层TEVAR术后出现急性肾损伤的临床风险及意义尚无广泛研究。本实验研究患者术后急性肾损伤的发生率为18.7%,与Piffaretti等[5]171例患者研究中,TEVAR术后急性肾损伤的发生率14%基本相符。Eggebrecht等[3, 10]报道TEVAR术后出现急性肾损伤分别为34%、11.4%。相关报道急性肾损伤的发生率存在一定差异,考虑主要与急性肾损伤定义所采用标准及研究人群的不同相关。
对于TEVAR术后出现急性肾损伤的危险因素,目前尚无一致结论。有研究[11]报道,患者入院高收缩压及双侧肾动脉受累是术前急性肾损伤发生的独立危险因素。入院患者的高收缩压,可能与肾动脉受累相关,肾动脉受累可激活肾素-血管紧张素系统导致血压升高[12]。除此之外,收缩压的升高,可以导致假腔持续扩张及延伸,加重肾动脉受累程度,从而导致肾缺血加重。所以入院的高收缩压是Stanford B型主动脉夹层患者术前出现急性肾损伤的危险因素。本研究回归分析我院193例患者数据表明,入院高的收缩压不仅是术前急性肾损伤的危险因素,同时也是术后出现急性肾损伤的独立危险因素。目前尚无相关文献关于此方面的报道,本研究急性肾损伤组中单侧或双侧肾动脉完全由假腔供血及肾脏显影欠佳21例(58.33%),而非急性肾损伤组26例(16.56%),存在明显差异,其原因可能患者入院后血压持续偏高,累及肾动脉程度加重,成为完全假腔供血,同时假腔多呈螺旋走形[13],以单侧完全假腔供血居多,术前可能Scr不会出现明显上升,而TEVAR术后部分肾脏血供起源于假腔患者术后肾灌注显著减低,可能与破口被覆膜支架遮盖后,原假腔血流较前减少,假腔内压力减低有关[14]。本研究对于主动脉夹层内膜撕脱累及肾动脉的数据分析中,本研究还发现,对于双侧肾动脉均受累其术后急性肾损伤的发生率明显升高。其原因包括两方面:(1)单侧肾动脉受累,对侧肾动脉血流正常,肾小球滤过率可维持在正常范围内,不会导致Scr水平升高;(2)夹层累及肾动脉可导致肾脏血供减少,行TEVAR术后绝大多数患者肾动脉血流可得到改善,但部分患者肾动脉完全由假腔供血且假腔远端破口较小,腔内隔绝术后真腔压力增加导致假腔受压,出现假腔血流量减少,从而导致术后肾动脉的血供不能得到改善甚至缺血加重可能。
本研究结果显示,TEVAR术中造影剂的用量是患者术后出现急性肾损伤的又一独立危险因素。本研究数据表明,急性肾损伤组患者术中使用造影剂用量明显高于非急性肾损伤组。Abujudeh等[15-16]的研究显示,短时间内大量造影剂的应用会使造影剂肾病(contrast induced nephropath,CIN)的发生率显著增加。造影剂对肾功能的影响主要是由于造影剂引起肾髓质缺血,同时也会使肾小管上皮变性、氧自由基产生增多均可导致患者术后急性肾功能不全。主动脉夹层患者中部分由于夹层存在多破口,术中需要大量造影剂来确定导丝及支架是否在TL及位置存是否适当,导致术后出现CIN。同时本研究中还证实,合并有糖尿病的患者,术后出现急性肾损伤明显高于无糖尿病病史患者。糖尿病在大多数试验中被认为是CIN的重要危险因素[17],无论患者既往是否合并肾功能不全,糖尿病患者造影剂使用后CIN发生率明显增高[18-19],有学者[20]称之为糖尿病造影剂急性肾损伤。有研究[19]发现,造影剂与高血糖具有协同作用,可促进肾细胞凋亡,导致急性肾损伤的发生。所以,在TBAD患者中合并糖尿病的患者,围手术期更应注重肾功能保护,对于合并糖尿病患者,做好术前心肾功能的评估以及疾病的相关评估,进行围术期水化治疗,控制造影剂的用量,术后严密的生命体征观察、准确的记录24 h出入量是预防术后CIN发生的重要措施。根据随访结果,急性肾损伤组患者早期病死率可达22.6%,明显高于非急性肾损伤组。所以对于TEVAR术后出现急性肾损伤的患者更应注重肾保护治疗,积极改善肾功能,可能对改善患者的预后具有重要意义。对于合并糖尿病病史患者,早期病死率明显高于无糖尿病病史患者。分析其中死亡患者原因,大多是由于血管动脉硬化出现心脑血管事件导致患者的死亡。糖尿病患者血糖异常,可引起机体脂代谢异常[21],而其代谢异常是动脉粥样硬化的主要原因。有研究[22-23]表明,糖尿病是脑卒中发生的独立危险因素。所以对于合并糖尿病病史的患者,围手术期及术后应严格控制血糖水平,可减少患者心脑血管事件的发生,改善患者预后。
腔内修复术治疗Stanford B型主动脉夹层是目前最为常规的治疗手段。有研究[24]显示,腔内修复术由于置入覆膜支架,属于异物置入,可激活体内炎症反应,导致患者出现体温增高、白细胞升高和凝血异常等。研究[25-26]报道,支架置入后的炎症反应并不一定都能自发消退,特别是对于高危患者可能诱发严重的全身炎症反应,进而导致急性肺损伤、急性肾功能不全等。本研究中比较两组患者入院白细胞水平及术后体温,两组间均无明显差异,这并不能说明炎症反应与患者术后出现急性肾损伤无相关性。查阅大量文献后发现,腔内修复术后,患者机体处于炎症高反应状态,更多表现为IL-10、IL-6、CRP水平的增高,由于本实验无法监测相关炎症指标,所以腔内隔绝术后患者急性肾损伤与炎症反应关系仍有待于进一步研究。
分析本研究存在问题,首先本研究是一项回顾性研究,部分患者由于病例资料及随访资料不全被剔除,可能会导致研究结果产生偏移。其次,本研究评估患者肾功能仅从Scr水平,由于尿量数据缺失,没有从术后尿量角度评估患者肾功能。最后,本研究总体病例数相对较少,其可靠性有待于大量病例进一步验证。
总结本研究结果,急性肾损伤是Stanford B型主动脉夹层行TEVAR术后常见的并发症。该研究证实,术后急性肾损伤的发生是患者早期预后的不利因素。入院高收缩压、术中造影剂用量、糖尿病病史、双侧肾动脉受累是TEVAR术后出现急性肾损伤的独立危险因素,同时糖尿病病史也会增加患者早期死亡风险。本研究对于Stanford B型主动脉夹层术后可能出现急性肾损伤高危患者的早期识别、早期预防、早期治疗具有重要的临床意义。
[1] Olsson C,Thelin S,Ståhle E,et al.Thoracic aortic aneurysm and dissection:increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002[J].Circulation,2006,114(24):2611-2618.doi:10.1161/CIRCULATIONAHA.106.630400.
[2] Nienaber CA,Clough RE.Management of acute aortic dissection[J].Lancet,2015,385(9970):800-811.doi:10.1016/S0140-6736(14)61005-9.
[3] Eggebrecht H,Breuckmann F,Martini S,et al.Frequency and outcomes of acute renal failure following thoracic aortic stent-graft placement [J].Am Cardiol,2006,98(4):458-463.doi:10.1016/j.amjcard.2006.02.052.
[4] Fairman RM,Criado F,Farber M,et al.Pivotal results of the Medtronic Vascular Talent Thoracic Stent Graft System:the VALOR trial [J].J Vasc Surg,2008,48(3):546-554.doi:10.1016/j.jvs.2008.03.061.
[5] Piffaretti G,Mariscalco G,Bonardelli S,et al.Predictors and outcomes of acute kidney injury after thoracic aortic endograft repair[J].J Vasc Surg,2012,56(6):1527-1534.doi:10.1016/j.jvs.2012.05.106.
[6] Ronco C,Levin A,Warnock DG,et al.Improving outcomes from acute kidney injury(AKI):Report on an initiative[J].Int J Artif Organs,2007,30(5):373-376.
[7] 竺挺,符伟国,王玉琦.复杂主动脉瘤及主动脉夹层的腔内治疗策略[J].中华外科杂志,2011,49(6):491-494.doi:10.3760/cma.j.issn.0529-5815.2011.06.004.Zhu T,Fu WG,Wang YQ.The endovascular treatment strategy of complex aortic aneurysm and aortic dissection[J].Chinese Journal of Surgery,2011,49(6):491-494.doi:10.3760/cma.j.issn.0529-5815.2011.06.004.
[8] 林长泼,符伟国.主动脉夹层的治疗进展[J].中国普通外科杂志,2016,25(6):790-794.Lin CP,Fu WG.Advances in treatment of aortic dissection[J].Chinese Journal of General Surgery,2016,25(6):790-794.
[9] Zhu JC,Chen SL,Jin GZ,et al.Acute renal injury after thoracic endovascular aortic repair of Stanford type B aortic dissection:incidence,risk factors,and prognosis[J].J Formos Med Assoc,2014,113(9):612-619.doi:10.1016/j.jfma.2014.01.017.
[10] Pisimisis GT,Khoynezhad A,Bashir K,et al.Incidence and risk factors of renal dysfunction after thoracic endovascular aortic repair[J].J Thorac Cardiovasc Surg,2010,140(6 Suppl):S161-167.doi:10.1016/j.jtcvs.2010.10.014.
[11] Ren HM,Wang X,Hu CY,et al.Relationship between acute kidney injury before thoracic endovascular aneurysm repair and inhospital outcomes in patients with type B acute aortic dissection[J].J Geriatr Cardiol,2015,12(3):232-238.doi:10.11909/j.issn.1671-5411.2015.03.002.
[12] 任红梅,王晓,栾红,等.急性主动脉夹层术前急性肾损伤危险因素分析[J].中国心血管病研究,2017,15(1):51-55.doi:10.3969/j.issn.1672-5301.2017.01.015.Ren HM,Wang X,Luan H,et al.Preoperative acute kidney injury in acute aortic dissection-risk factors analysis[J].Chinese Journal of Cardiovascular Research,2017,15(1):51-55.doi:10.3969/j.issn.1672-5301.2017.01.015.
[13] 肖子亚,王豪俊,姚晨玲,等.主动脉夹层患者多层螺旋CT血管成像表现及其与院内死亡的关系[J].中华心血管病杂志,2017,45(3):217-222.doi:10.3760/cma.j.issn.0253-3758.2017.03.009.Xiao ZY,Wang HJ,Yao CL,et al.Relationship between multi-slice spiral CT angiography imaging features and inhospital death of patients with aortic dissection[J].Chinese Journal of Cardiology,2017,45(3):217-222.doi:10.3760/cma.j.issn.0253-3758.2017.03.009.
[14] 刘东婷,刘家祎,温兆赢,等.320排容积CT对主动脉夹层患者手术前后肾脏血流灌注改变的初步研究[J].心肺血管病杂志,2016,35(12):967-973.doi:10.3969/j.issn.1007-5062.2016.12.010.Liu DT,Liu JY,Wen ZY,et al.Preliminary study of the application of 320-slice dynamic volume CT on the changes of renal blood flow before and after operation in patients with aortic dissection[J].Journal of Cardiovascular and Pulmonary Diseases,2016,35(12):967-973.doi:10.3969/j.issn.1007-5062.2016.12.010.
[15] Abujudeh HH,Gee MS,Kaewlai R.In emergency situations,should serum creatinine be checked in all patients before performing second contrast CT examinations within 24 hours?[J].J Am Coll Radiol,2009,6(4):268-273.doi:10.1016/j.jacr.2008.09.014.
[16] Yang JS,Peng YR,Tsai SC,et al.The molecular mechanism of contrast-induced nephropathy(CIN)and its link to in vitro studies on iodinated contrast media(CM)[J].Biomedicine(Taipei),2018,8(1):1.doi:10.1051/bmdcn/2018080101.
[17] Stolker JM,McCullough PA,Rao S,et al.Pre-procedural glucose levels and the risk for contrast-induced acute kidney injury in patients undergoing coronary angiography[J].J Am Coll Cardiol,2010,55(14):1433-1440.doi:10.1016/j.jacc.2009.09.072.
[18] 万立野,李宝群,毕红东,等.等渗及低渗造影剂对糖尿病合并轻中度肾功能不全患者肾毒性的对比研究[J].实用医学杂志,2016,32(22):3799-3800.doi:10.3969/j.issn.1006-5725.2016.22.049.Wan LY,Li BQ,Bi HD,et al.Comparative study of nephrotoxicity of isotonic and hypotonic contrast agents in diabetic patients with mild or moderate renal failure[J].The Journal of Practical Medicine,2016,32(22):3799-3800.doi:10.3969/j.issn.1006-5725.2016.22.049.
[19] Tan H,Yi H,Zhao W,et al.Intraglomerular crosstalk elaborately regulates podocyte injury and repair in diabetic patients:insights from a 3D multiscale modeling study[J].Oncotarget,2016,7(45):73130-73146.doi:10.18632/oncotarget.12233.
[20] 李香玲,孙桂玲,郭民.促红细胞生成素对糖尿病大鼠造影剂急性肾损伤的影响[J].现代预防医学,2015,42(2):316-319.Li XL,Sun GL,Guo M.The in fluence of hemopoietin on contrast medium-induced acute kidney injury in diabetic rats[J].Modern Preventive Medicine,2015,42(2):316-319.
[21] 景良洪,曾艳丽,宋凤平.综合护理干预对2型糖尿病患者血糖血脂水平及心脑血管事件的影响[J].检验医学与临床,2016,13(12):1712-1714.doi:10.3969/j.issn.1672-9455.2016.12.049.Jing LH,Zeng YL,Song FP.Effect of comprehensive nursing intervention on levels of blood glucose and lipid as well as cardiovascular and cerebrovascular events in patients with type 2 diabetes[J].Laboratory Medicine and Clinic,2016,13(12):1712-1714.doi:10.3969/j.issn.1672-9455.2016.12.049.
[22] Sung YF,Lee JT,Tsai CL,et a1.Risk Factor Stratification for Intracranial Stenosis in Taiwanese Patients With Cervicocerebral Stenosis [J].J Am Heart Assoc,2015,4(12).pii:e002692.doi:10.1161/JAHA.115.002692.
[23] Mostaza JM,Lahoz C,Salinero-Fort MA,et a1.Carotid atherosclerosis severity in relation to glycemic status:a crosssectional population study[J].Atherosclerosis,2015,242(2):377-382.doi:10.1016/j.atherosclerosis.2015.07.028.
[24] Moulakakis KG,Sfyroeras GS,Papapetrou A,et al.In flammatory response and renal function following endovascular repair of the descending thoracic aorta[J].J Endovasc Ther,2015,22(2):201-206.doi:10.1177/1526602815573227.
[25] Moulakakis KG,Alepaki M,Sfyroeras GS,et al.The impact of endograft type on inflammatory response after endovascular treatment of abdominal aortic aneurysm[J].J Vasc Surg,2013,57(3):668-677.doi:10.1016/j.jvs.2012.09.034.
[26] Toya N,Ohki T,Momokawa Y,et al.Risk factors for early renal dysfunction following endovascular aortic aneurysm repair and its effect on the postoperative outcome[J].Surg Today,2016,46(12):1362-1369.doi:10.1007/s00595-016-1324-6.
