动脉狭窄性疾病的治疗理念处于不断更新中,但自体静脉移植,仍然是重建冠脉及外周动脉的主要方式[1-2]。移植静脉狭窄(vein graft stenosis,VGS)限制了其远期疗效。但是,VGS的确切机制尚未阐明[3]。之前关于VGS的研究都基于一个假设:移植静脉的内膜增生,同动脉损伤后的内膜增生,具有相似的病理生理过程[4-6]。但是,基于上述思路的临床实验并未改善移植静脉的远期通畅率[7-8]。
发育生物学研究提示:胚胎期,循环系统建立之初,动脉与静脉就存在基因水平的差异[9]。ephrinB2及其受体EphB4这一对分子标记物是血管形成过程中动、静脉分化的决定性分子,促使初期脉管分别向动脉、静脉分化。在成年动物,两者分别分布于动脉、静脉的内皮细胞,维持成年动物动、静脉的结构及功能稳定,是动、静脉的分子指纹[10]。
前期研究[5, 11]提示:移植静脉适应动脉血流的过程具有其静脉特异性,可能与动脉损伤后的狭窄存在分子水平的差异。本研究采用成组设计,观察不同EphB4表达量对移植静脉吻合口管腔、内皮细胞迁移能力、ERK1/2(细胞外信号调节激酶途径)通路活化的影响,探索EphB4相关、静脉特异性的适应动脉血流的调控机制,从全新的视野为VGS的防治提供理论依据。
1 材料与方法
1.1 动物实验
1.1.1 实验动物 成年雄性野生型SD大鼠50只,体质量220~300 g,由昆明医科大学SPF实验中心提供。成年雄性EphB4+/-型转基因SD大鼠10只,体质量220~300 g,由广州赛业生物科技有限公司制备,昆明医科大学SPF实验中心繁育。大鼠均在“昆明医科大学实验动物饲养与操作规程”规范下进行实验操作。
1.1.2 移植静脉模型建立 2%戊巴比妥钠腹腔注射麻醉大鼠,供体大鼠行胸部正中切口,剪取上腔静脉约2 cm,置于肝素生理盐水中备用。受体大鼠行腹部正中切口,暴露大鼠腹主动脉及下腔静脉并肝素化,血管夹阻断主动脉近远端血流,离断腹主动脉,将供体上腔静脉以端端吻合方式,移植到受体腹主动脉,重建动脉血流[12]。
1.1.3 分组及处理 单纯模型组:随机选择野生型大鼠20只(供体、受体各10只),建立移植静脉动物模型;EphB4增强组:将ephrinB2-Fc溶于PluronicF127胶与阳离子聚合物PEI的混合液中,配置为1 µg/mL;随机选择野生型大鼠20只(供体、受体各10只),建模后取混合物200 μL涂抹于移植静脉外膜[4];EphB4减弱组:随机选野生型大鼠10只,EphB4+/-型大鼠10只,将EphB4+/-型转基因大鼠静脉行端端吻合,移植到野生型大鼠腹主动脉。按取材时间不同,各组内再分为术后1周组及术后4周组。
1.1.4 标本的收集与处理 过量麻醉处死大鼠后,切取移植静脉,置于4%多聚甲醛中过夜,石蜡包埋,制片备用。分别行:HE染色、α-平滑肌肌动蛋白(α-actin)免疫组织化学染色、Masson染色,并测量移植静脉内膜+中膜厚度。
1.2 细胞实验
1.2.1 细胞来源 野生型大鼠静脉内皮细胞取自大鼠上腔静脉,EphB4+/-型大鼠静脉内皮细胞取自EphB4+/-型大鼠上腔静脉。
1.2.2 干预措施 以相同浓度的ephrinB2-Fc分别刺激野生型及EphB4+/-型静脉内皮细胞进行qRT-PCR对比两种细胞EphB4 mRNA的表达,进行Western blot对比两种细胞p-ERK1/2、磷酸化EphB4膜受体(p-EphB4R)表达的异同,进行免疫荧光、细胞划痕实验。
1.3 实验试剂
大鼠ephrinB2-Fc蛋白购于Sino-biological公司,α-actin单克隆抗体、EphB4单克隆抗体,免疫组化染色试剂盒,免疫荧光染色试剂盒,PCR试剂盒等,均购于Cell signaling technology公司。
1.4 具体实验方法
HE染色,平滑肌细胞特异性染色(α-actin)及胶原纤维特异性染色Massion染色,由云南省阜外心血管病医院病理科按相关操作规程进行。RT-PCR、Western blot、免疫荧光染色均按相关试剂盒操作规程完成。α-actin及Massion染色结果判定:在光强度及曝光度相同的条件下,每例切片在10×40光学显微镜下任选5个视野,测定其阳性颗粒的平均光密度值(optical density,OD),以此代表免疫组化阳性细胞的量。荧光染色结果判定:依照细胞阳性着色程度,可分为:弱阳性(+)1分;中等阳性(++)2分;强阳性(+++)3分。采用积分综合计量。计算公式为:(+)%×1+(++)%×2+(+++)%×3;总数值<0.5者为(-),<1.0者为(+),1.0~1.5者为(++),>1.5者为(+++);至少随机观察5~10个视野。细胞划痕实验:培养内皮细胞,中央区域划线,不同时间观察周边细胞是否移动至中央划痕区,以此判断细胞迁移能力。
1.5 图像分析及统计学处理
应用NIH Image J医学图象分析系统对免疫组化染色后的图像进行定量分析。数据以均数±标准差( ±s)表示,应用SPSS 22.0统计软件分析,组间比较采用单因素方差分析,P<0.05为差异有统计学意义。
±s)表示,应用SPSS 22.0统计软件分析,组间比较采用单因素方差分析,P<0.05为差异有统计学意义。
2 结 果
2.1 组织病理学结果
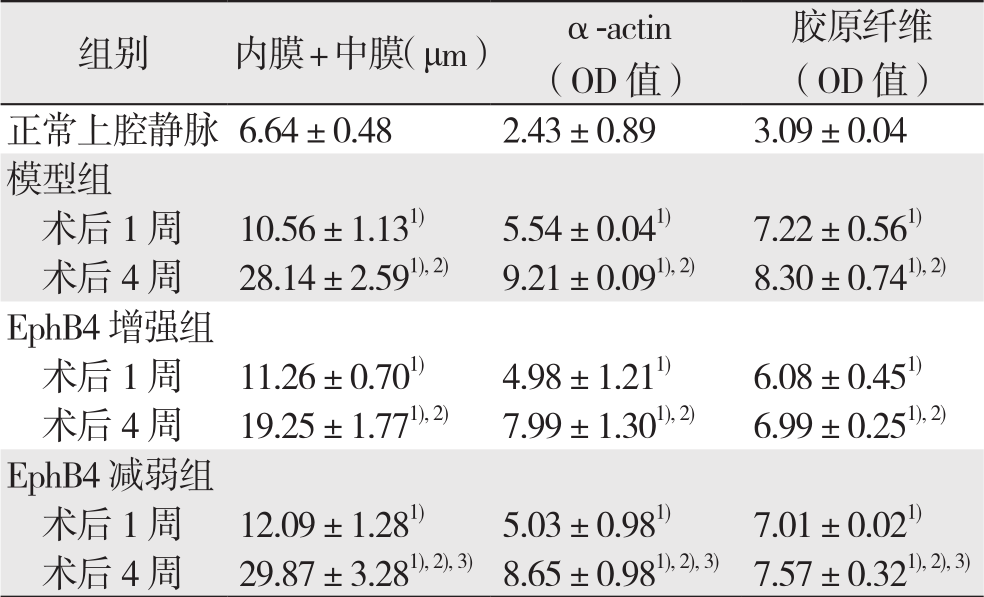
建模成功后1、4周取材(共4组,n=5)。通过HE染色测量内膜+中膜厚度(图1),结果提示:与正常上腔静脉比较,各实验组移植静脉的内膜+中膜厚度均明显增加(均P<0.05);术后1周,各实验组组间内膜+中膜厚度差异无统计学意义(P>0.05);术后4周,各组内膜+中膜厚度组间比较出现明显差异(P<0.05)。其中,ephrinB2增强组内膜+中膜厚度明显低于模型组,而EphB4减弱组内膜+中膜厚度明显高于ephrinB2增强组(均P<0.05),但EphB4减弱组与模型组间差异未达统计学意义(P>0.05)。α-actin及Massion染色结果显示:术后4周,ephrinB2干预组α-actin阳性细胞及胶原纤维表达,明显低于对照组,而EphB减弱组则高于ephrinB2增强组(图1)(表1)(均P<0.05)。

图1 大鼠正常上腔静脉与术后4周各组移植静脉标本染色结果(×100)
Figure 1 Staining results of the normal rat superior vena cava and the vein graft s od each experimental group at 4 weeks aft er model creation(×100)
表1 大鼠正常上腔静脉与各实验组内膜+中膜厚度、α-actin及胶原纤维含量比较( ±s)
±s)
Table 1 Comparison of the intima-media thickness,and the contents of smooth muscle actin and collagen fiber among normal rat superior vena cava and the experimental groups( ±s)
±s)
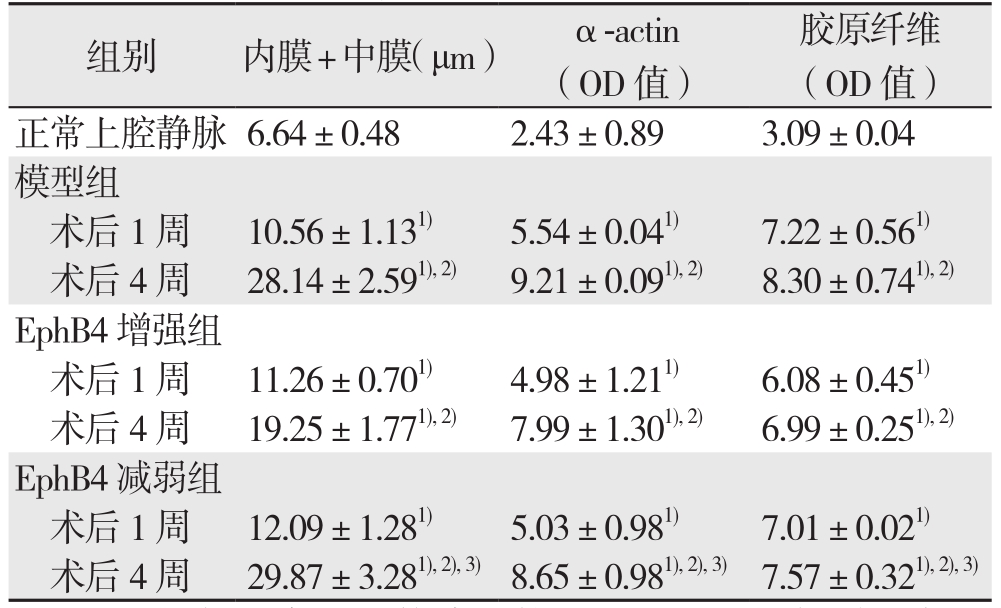
注:1)与正常上腔静脉比较,P<0.05;2)与同组术后1周比较,P<0.05;3)与EphB4增强组比较,P<0.05
Note:1)P<0.05 vs. normal rat superior vena cava; 2)P<0.05 vs. same group at 1 week aft er model creation; 3)P<0.05 vs. EphB4 enhancing group
2.2 磷酸化水平检测结果
相同浓度ephrinB2-Fc(1 μg/mL)分别刺激野生型(EphB4+/+)及EphB4+/-型静脉内皮细胞,免疫荧光与Western blot结果均显示,EphB4+/-型静脉内皮细胞ERK1/2、EphB4R磷酸化程度弱于野生型(图2);qRT-PCR结果显示,EphB4+/-型静脉内皮细胞的EphB4 mRNA转录活性明显低于野生型(P<0.05)(图3)。
2.3 细胞迁移能力检测结果
细胞划痕实验结果显示,两种内皮细胞在同等条件、相同浓度ephrinB2-Fc(1 μg/mL,6 h)刺激下,EphB4+/-型静脉内皮细胞迁移程度明显低于野生型(P<0.05)(图4)。
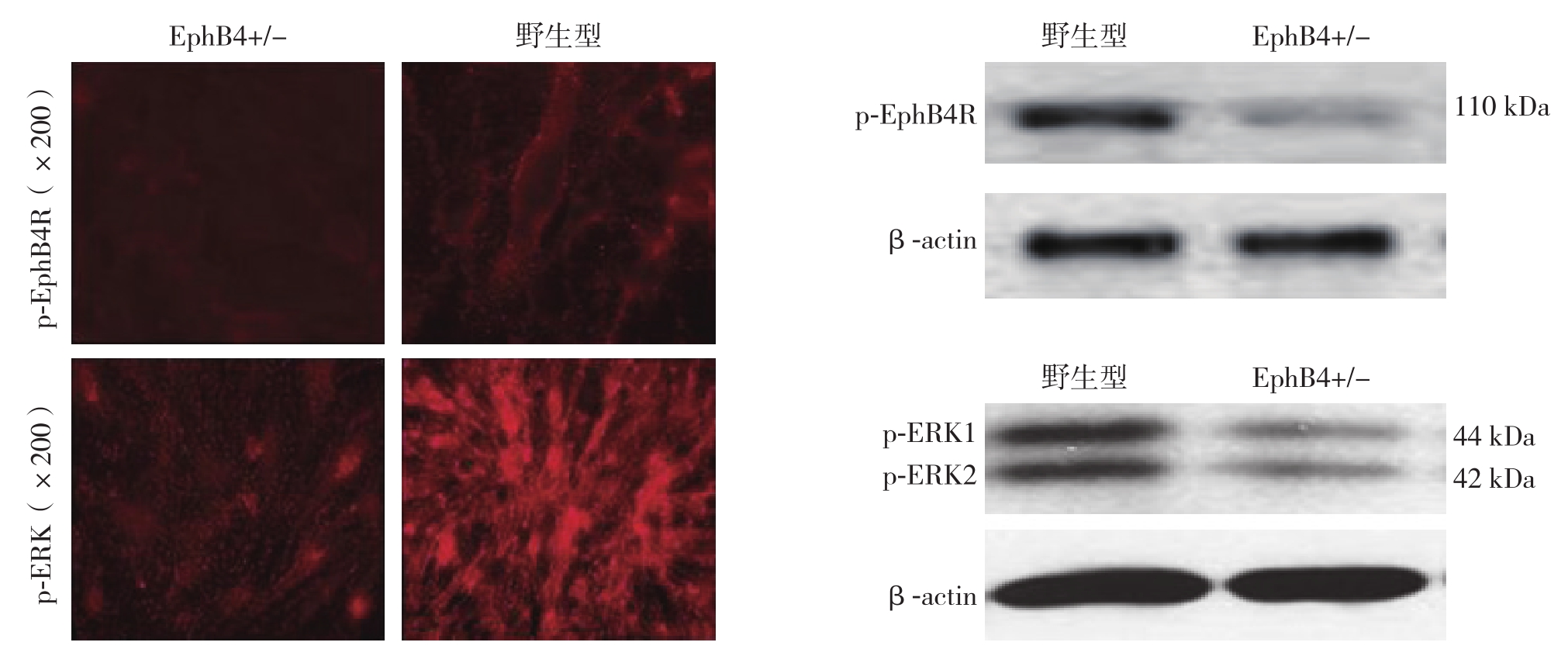
图2 野生型与EphB4+/-型大鼠静脉内皮细胞ERK1/2、EphB4R磷酸化程度比较
Figure 2 Comparison of the degrees of phosphorylation of EphBR and ERK1/2 between vein endothelial cells from wild type and EphB4+/- type rats
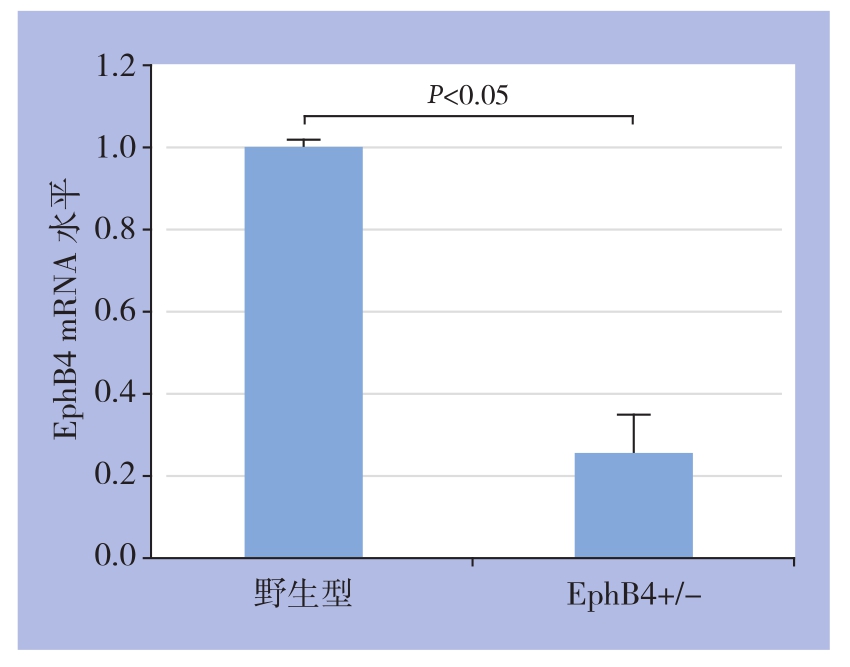
图3 野生型与EphB4+/-型大鼠静脉内皮细胞mRNA转录活性比较
Figure 3 Figure 2 Comparison of the transcriptional activityies of EphB4 mRNA between vein endothelial cells from wild type and EphB4+/- type rats
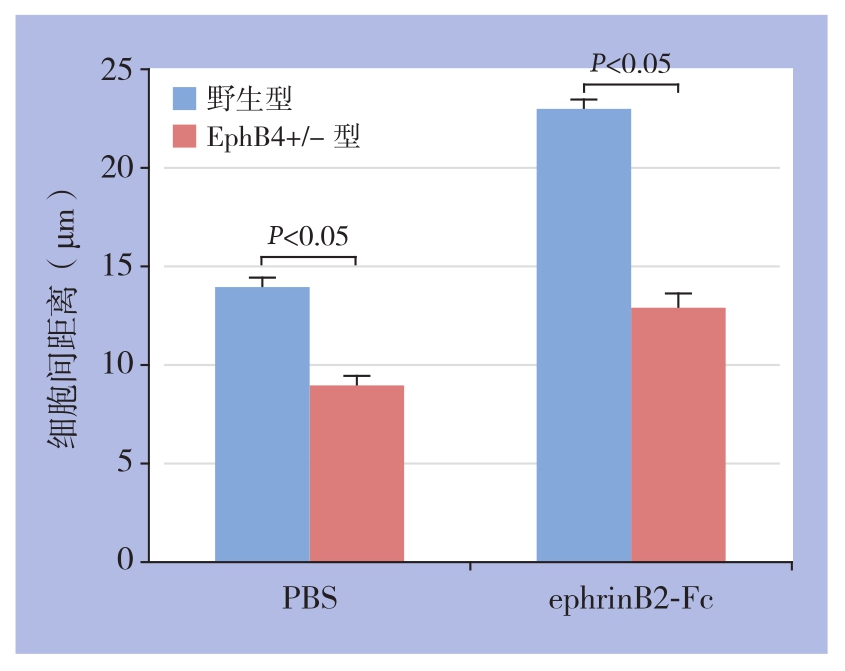
图4 细胞划痕实验结果
Figure 4 Results of scratch-wound assay
3 讨 论
无论是静脉移植重建动脉[13],还是血管介入治疗腔内重建动脉[14-15],其共同的瓶颈就是术后再狭窄。这一问题一直是心血管病内、外科医生面临的重要挑战,此过程所特有的复杂机制尚无任何学说能够做出清楚的解释。
动脉术后再狭窄的共同通路是:血管内膜高度增生[16-17]。由于动、静脉的内膜增生有着相似的病理生理过程和临床结局,过去的研究都是基于同一个假设,也就是动、静脉的内膜增生拥有共同机制。处理动脉内膜增生的治疗策略,同样被应用于处理移植静脉的内膜增生。可以概括为:利用不同载体,将不同的治疗因子转染致静脉移植物管壁,或直接利用药物涂层支架[18]或者涂层球囊[19],改善静脉移植物或介入治疗后动脉的远期通畅率,但是运用于临床,未能达到预期的治疗效果。
基于发育生物学及课题组前期研究结果[20-21],本研究的假设是:成年动物,静脉具有可塑性,静脉分子指纹EphB4的表达量,可能是移植静脉内膜增生的核心调节因子。保证EphB4的表达,可维持静脉结构及功能的“稳态”,可能将减少移植物的内膜增生及管腔狭窄。研究从动物实验和细胞实验两方面验证了假设。静脉移植术后4周,EphB4增强组的内膜+中膜厚度,平滑肌细胞增殖程度均较EphB4减弱组及模型组显著减少。从组织学层面提示EphB4表达量的差异对内膜增生的影响。细胞学研究进一步验证EphB4表达量不同时,EphB4膜受体磷酸化,下游传导通路ERK1/2的激活程度不同。划痕实验模拟了内皮细胞的迁移,EphB4+/-型静脉内皮细胞迁移程度显著低于野生型,推测基因敲除后的内皮细胞功能弱化。虽然细胞实验不能完全反映动物实验中内膜增生及管壁重塑的机制,但可以推测:内皮细胞功能的减弱,可能是导致移植静脉管壁增厚的原因之一。ERK1/2信号通路与EphB4信号通路有密切关联[22-23],且同时满足以下条件:(1)同EphB4一样在静脉源细胞中表达;(2)在VGA过程中,其蛋白表达的改变与移植静脉内膜增生过程密切相关[24];(3)ERK1/2与Eph家族有交互作用[25]。通过ERK通路阻断实验,进一步证实了ERK1/2在EphB4主导的调控过程中所起的作用。
通过实验验证了静脉特异性内膜增生的机制之后,下一步的工作将集中对动脉特异性的内膜增生进行研究,从一个新的角度来探索动、静脉内膜增生的分子本质差异。移植静脉适应动脉血流的过程中,保证静脉指纹分子EphB4的表达,可能保持了移植静脉的静脉属性,减轻血管重塑,改善移植静脉狭窄;EphB4-ERK1/2在静脉特异性的调控机制中的可能通过控制EphB4的表达而起主要作用。
[1] Chlupác J,Filová E,Bacáková L.Blood vessel replacement:50 years of development and tissue engineering paradigms in vascular surgery[J].Physiol Res,2009,58(Suppl 2):S119-139.
[2] Lei M,Giddens DP,Jones SA,et al.Pulsatile flow in an endto-side vascular graft model:comparison of computations with experimental data[J].J Biomech Eng,2001,123(1):80-87.
[3] Owens CD.Adaptive changes in autogenous vein grafts for arterial reconstruction:clinical implication[J].J Vasc Surg,2010,51(3):736-746.doi:10.1016/j.jvs.2009.07.102.
[4] Borin TF,Miyakawa AA,Cardoso L,et al.Apoptosis,cell proliferation and modulation of cyclin-dependent kinase inhibitor p21(cip1)in vascular remodelling during vein arterialization in the rat[J].Int J Exp Pathol,2009,90(3):328-337.doi:10.1111/j.1365-2613.2009.00648.x.
[5] Kudo FA,Mute A,Maloney SP,et al.Venous identity is lost but arterial identity is not gained during vein graft adaptation[J].Arterioscler Thromb Vasc Biol,2007,27(7):1562-1571.doi:10.1161/ATVBAHA.107.143032
[6] 王清宇,徐在品,赵伟,等.ePTFE套管对犬移植静脉动脉化的影响[J].中国普通外科杂志,2009,18(12):1271-1274.Wang QY,Xu ZP,Zhao W,et al.Effects of ePTFE cuff on arterialization of implanted vein in dogs[J].Chinese Journal of General Surgery,2009,18(12):1271-1274.
[7] Conte MS,Bandyk DF,Clowes AW,et al.Results of PREVENT III:a multicenter,randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery[J].J Vasc Surg,2006,43(1):742-751.doi:10.1016/j.jvs.2005.12.058.
[8] Alexander JH,Ha fley G,Harrington RA,et al.Efficacy and safety of edifoligide,an E2F transcription factor decoy,for prevention of vein graft failure following coronary artery bypass graft surgery:PREVENT IV:a randomized controlled trial[J].JAMA,2005,294(2):2446-2454.doi:10.1001/jama.294.19.2446
[9] Wang Z,Cohen K,Shao Y,et al.Ephrin receptor,EphB4,regulates ES cell differentiation of primitive mammalian hemangioblasts,blood,cardiomyocytes,and blood vessels[J].Blood,2004,103(1):100-109.doi:10.1182/blood-2003-04-1063.
[10] Adams RH,Wilkinson GA,Weiss C,et al.Roles of ephrinB ligands and EphB receptors in cardiovascular development:demarcation of arterial /venous domains,vascular morphogenesis,and sprouting angiogenesis[J].Genes Dev,1999,13(3):295-306.
[11] Rivera M,Mute A,Feifel A,et al.Venous and arterial identity:a role for caveolae?[J].Vascular,2009,17(Suppl 1):S10-14.
[12] 苏刚,孙宗全,董念国,等.大鼠自体移植静脉内膜增生模型的改进[J].山东医药,2006,46(33):9-10.doi:10.3969/j.issn.1002-266X.2006.33.005.Su G,Sun ZQ,Dong NG,et al.Improvement of intimal hyperplasia model of autologous vein graf in rat[J].Shandong Medical Journal,2006,46(33):9-10.doi:10.3969/j.issn.1002-266X.2006.33.005.
[13] Collins MJ,Li X,Lv W,et al.Therapeutic strategies to combat neointimal hyperplasia in vascular grafts[J].Expert Rev Cardiovasc Ther,2012,10(5):635-647.doi:10.1586/erc.12.33.
[14] 吴元兵,朱云峰,葛红卫,等.下肢动脉硬化闭塞症腔内治疗后支架内再狭窄的治疗[J].中国普通外科杂志,2015,24(12):1687-1690.doi:10.3978/j.issn.1005-6947.2015.12.010.Wu YB,Zhu YF,Ge HW,et al.Management of in-stent restenosis in lower limb arteriosclerosis obliterans after endovascular treatment[J].Chinese Journal of General Surgery,2015,24(12):1687-1690.doi:10.3978/j.issn.1005-6947.2015.12.010.
[15] 刘永东,苏兴旺,孙立伶,等.腔内治疗下肢动脉硬化闭塞症后支架内再狭窄的治疗[J].海南医学,2017,28(21):3555-3557.doi:10.3969/j.issn.1003-6350.2017.21.037.Liu YD,Su XW,Sun LL,et al.Treatment of stent restenosis after endovascular treatment of arteriosclerosis obliterans of lower extremity[J].Hainan Medical Journal,2017,28(21):3555-3557.doi:10.3969/j.issn.1003-6350.2017.21.037.
[16] 李海明,顾承雄.静脉桥血管内膜增生机制的研究进展[J].心肺血管病杂志,2017,36(3):237-239.doi:10.3969/j.issn.1007-5062.2017.03.023.Li HM,Gu CX.Research advances in endothelial regeneration of vein grafts[J].Journal of Cardiovascular and Pulmonary Diseases,2017,36(3):237-239.doi:10.3969/j.issn.1007-5062.2017.03.023.
[17] Lajos TZ,Robicsek F,Thubrikar M,et al.Improving patency of coronary conduits "valveless" veins and/or arterial grafts[J].J Card Surg,2007,22(2):170-177.doi:10.1111/j.1540-8191.2007.00380.x.
[18] 吴中俭,谷涌泉,齐立行,等.单纯球囊扩张与联合药物涂层支架置入治疗膝下动脉闭塞性疾病的效果比较[J].中国医药导报,2017,14(22):66-68.Wu ZJ,Gu YQ,Qi LX,et al.Comparison the effect of balloon dilatation alone and combination with drug eluting stent implantation in treatment of infrapopliteal arterial occlusive disease[J].China Medical Herald,2017,14(22):66-68.
[19] Xu B,Qian J,Ge J,et al.Two-year results and subgroup analyses of the PEPCAD China in-stent restenosis trial:A prospective,multicenter,randomized trial for the treatment of drug-eluting stent in-stent restenosis[J].Catheter Cardiovasc Interv,2016,87(Suppl 1):624-629.doi:10.1002/ccd.26401.
[20] Kume T.Speci fication of arterial,venous,and lymphatic endothelial cells during embryonic development[J].Histol Histopathol,2010,25(5):637-646.doi:10.14670/HH-25.637.
[21] Wang Hu,Chen ZF,Anderson DJ.Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4[J].Cell,1998,93(5):741-753.
[22] Yang C,Guo Y,Jadlowiec CC,et al.Vascular endothelial growth factor-A inhibits EphB4 and stimulates delta-like ligand 4 expression in adult endothelial cells[J].J Surg Res,2013,183(1):478-486.doi:10.1016/j.jss.2013.01.009.
[23] 郭媛媛.VEGF-A对静脉内细胞分子标记物EphB4表达的调控及其抑制移植静脉内膜增生的机制[D].长沙:中南大学,2012.Guo YY.The mechanism of VEGF inhibit vein graft intimal hyperplasia by regulating venous marker EphB4 expression[D].Changsha:Central South University,2012.
[24] Gulkarov I,Bohmann k,Cinnante KM,et al.Topical mitogenactivated protein kinases inhibition reduces intimal hyperplasia inarterialized vein grafts[J].J Surg Res,2009,154(1):150-156.doi:10.1016/j.jss.2008.04.025.
[25] Haupaix N,StolfiA,Sirour C,et al.p120RasGAP mediates ephrin/Eph-dependent attenuation of FGF/ERK signals during cell fate speci fication in ascidian embryos[J].Development,2013,140(21)4347-4352.
