胰十二指肠切除手术(pancreaticoduodenectomy,PD)为治疗胰头部、Vater壶腹周围良恶性肿瘤的标准手术方式[1]。该手术自1945年由Whipple[2]第一次报道以来,通过不断改进手术技艺、加强围手术期管理,目前术后病死率已降至5%以下[3],但是以术后胰瘘(postoperative pancreatic fistula,POPF)为主的并发症发生率却依然高达40%~50%[1,4],导致患者住院时间与医疗费用均明显增加[5]。近几年来有研究发现PD术后第1天腹腔引流液淀粉酶水平(drain fluid amylase on postoperative day 1,DFA1)能较准确预测术后胰瘘发生[6-8],对于胰瘘低风险的患者,可采取术后早期拔除腹腔引流管、早期下床活动、早期进食等快速康复措施,以降低术后并发症发生率,促进患者快速康复[9-10]。但目前国际上DFA1预测界值始终争议较大,从90 U/L到5 000 U/L均有报道[6-8,11-12],且国内相关数据较少。因此,本研究回顾性分析了西南医院肝胆外科2013年1月—2015年10月收治并符合标准的PD手术患者的临床资料,试图通过统计分析计算出能够准确预测PD术后早期胰瘘发生的DFA1截断值,为患者术后实施快速康复提供指导与帮助。
1 资料与方法
1.1 患者纳入排除标准
患者纳入标准:⑴ 因胰头部、Vater壶腹周围良恶性肿瘤行PD手术的患者,手术方式包括保留幽门胰十二指肠切除术(pylorus preserving pancreaticoduodenectomy,PPPD)和经典Whipple手术;⑵ KPS评分>50分[13],能耐受麻醉和手术的患者。患者排除标准:⑴ 既往有上腹部手术史(经腹腔镜胆囊切除术除外)患者;⑵ 术前合并严重心血管、呼吸系统等疾病的患者;⑶ 术前影像学检查或术中探查肿瘤有远处转移的患者;⑷ 术中联合其它脏器切除的患者;⑸ 手术在腹腔镜或机器人辅助下实施的患者。
1.2 一般资料
本研究共回顾分析PD手术患者430例,男284例,女146例;年龄14~80岁,中位年龄58岁。术前合并糖尿病患者57例,高血压患者63例,有吸烟史患者1 7 9例。术后病理检查结果示:胰腺癌患者163例(37.9%),慢性胰腺炎患者65例(15.1%),胆总管腺癌患者38例(8.8%),十二指肠乳头部腺癌患者61例(14.2%),Vater壶腹部腺癌患者43例(10.0%),其它良恶性肿瘤患者60例(13.9%)。
1.3 手术及围手术期处理
所有患者术前全面检验、检查,包括上腹部CT血管造影或磁共振血管成像,60岁以上或既往有呼吸系统疾病患者予以动脉血气分析及肺功能检查,以全面评估病情、制定手术方案。手术均由我科具有副高级职称以上的医师主刀施行,结合术前影像学检查及术中情况选择行经典Whipple手术或保留幽门胰十二指肠切除术,消化道重建均采用Child方式,残端胰腺吻合均采用胰腺导管对空肠黏膜端侧吻合,并放置胰管内支架行胰液引流,其中2例患者因残余胰腺明显萎缩、外分泌功能基本丧失而缝闭胰腺残端。术中记录手术时间、出血量及红细胞等血制品输入量,残端胰腺质地由主刀医师触诊判断,测量主胰管直径,手术结束前于胰肠吻合旁和Winslow孔分别放置1根硅胶引流管从腹壁引出,切除肿瘤标本均送病理切片检查。术后予以24 h生命体征监护、呼吸道和腹腔引流管护理、抗生素预防感染和生长抑素类似物抑制胰腺外分泌等治疗方案;监测血常规、电解质、肝肾功能以及术后第1、3、5天腹腔引流液与血清淀粉酶水平,其中腹腔引流液淀粉酶取当日2根引流管最高值。记录胰肠吻合旁、Winslow孔引流管拔除时间,2根引流管全部拔除时间记为腹腔引流管拔除时间。
1.4 术后并发症的诊断
⑴ 术后胰瘘:参考国际胰瘘研究组织关于术后胰瘘的定义:PD术后第3天或以后,任何可计量的腹腔引流液中淀粉酶含量高于正常血清淀粉酶上限值的3倍,称为术后胰瘘,并根据严重程度分为A、B、C 3级[14]。本研究中进一步将PD术后5 d内发生的胰瘘称为术后早期胰瘘(early POPF)。⑵ 术后出血:分为腹腔内出血与消化道出血,患者血红蛋白浓度下降>3 g/dL,须行容量复苏并输注2个单位以上红细胞补充血容量,严重时甚至须采用血管栓塞或手术止血等方法予以治疗[15-17]。⑶ 术后胆汁漏:术后第3天或以后腹腔引流液中胆红素浓度高于血清胆红素浓度上限值的3倍,或者因为腹腔胆汁集聚、胆汁性腹膜炎须行穿刺引流、手术治疗等情况[18]。⑷ 腹腔脓肿:经超声或CT证实的腹腔积液,且微生物培养阳性,伴有持续发热及血清白细胞升高[19]。⑸ 腹腔感染:有临床体征,腹腔引流液微生物培养阳性,但未形成脓肿[19]。⑹ 胃排空延迟:术后7 d后仍未能恢复进食,且需要延长鼻胃管留置时间[20]。⑺ 肺部感染:发热伴血白细胞升高,有肺部X光片、CT扫描等影像学依据或痰液微生物培养呈阳性结果,须予以抗生素治疗[21]。
1.5 统计学处理
应用SPSS 17.0统计学软件对数据进行处理分析。计数资料以例数(百分比)[n(%)]表示,采用χ2检验进行比较,计量资料以中位数(范围)[M(范围)]表示,不符合正态分布采用非参数Mann-Whitney U检验进行比较,多因素分析采用二元Logistic回归模型,术后早期胰瘘的预测应用受试者操作特性曲线(receiver operating characteristic curve,ROC)进行分析。P<0.05为差异有统计学意义。
2 结 果
2.1 PD术后早期胰瘘与无早期胰瘘患者的情况分析
共纳入430例患者,术后发生早期胰瘘116例(26.9%),其中后期分别发展为A级胰瘘68例(58.6%),B级胰瘘41例(35.3%),C级胰瘘7例(6.1%)。未发生早期胰瘘的314例患者中,除外术后5 d内出现胆汁漏、腹腔引流血性液体、腹腔引流液浑浊以及术后第3天以后腹腔引流液量≥500 mL/d和超声确定胰周积液>5 cm的患者,剩余305例患者中,98例术后5 d内(术后3~5 d)拔除腹腔引流管(早期拔除组),207例术后5 d后(术后6~20 d)拔除腹腔引流管(延迟拔除组)。两组患者在基线资料比较,无统计学差异(表1-2)。术后5 d内拔除腹腔引流管不会增加术后腹腔穿刺引流、二次手术以及院内死亡的概率(均P>0.05),反而降低了术后胰瘘、腹腔感染以及总并发症发生率,且术后住院时间也明显缩短(均P<0.05)(表3)。
2.2 PD术后早期胰瘘发生的影响因素分析
将围手术期相关指标对PD术后早期胰瘘作单因素分析,结果显示:BMI、手术时间、残余胰腺质地、胰管直径、是否胰腺病变以及DFA1、术后第1天血清淀粉酶水平(serum amylase on postoperative day 1,SA1)、DFA1与SA1比值、D F A1与S A1差值为术后早期胰瘘的影响因素(表4-5);将上述影响因素代入非条件二元Logistic回归模型分析,得出DFA1、SA1为PD术后早期胰瘘的独立影响因素(表6)。
2.3 DFA1、SA1对PD术后早期胰瘘发生的预测分析
分别利用DFA1、SA1对PD术后早期胰瘘发生作ROC曲线图,得出曲线下面积(area under curve,AUC)分别为0.916和0.745(图1),并计算Youden指数确定DFA1、SA1预测PD术后早期胰瘘发生的截断值分别为494.75 IU/L、96.35 IU/L(表7)。
表1 早期拔管组与延迟拔除组患者基线资料(计数资料)比较[n(%)]
Table 1 Comparison of baseline data (enumeration data) between early tube removal group and delayed tube removal group [n (%)]
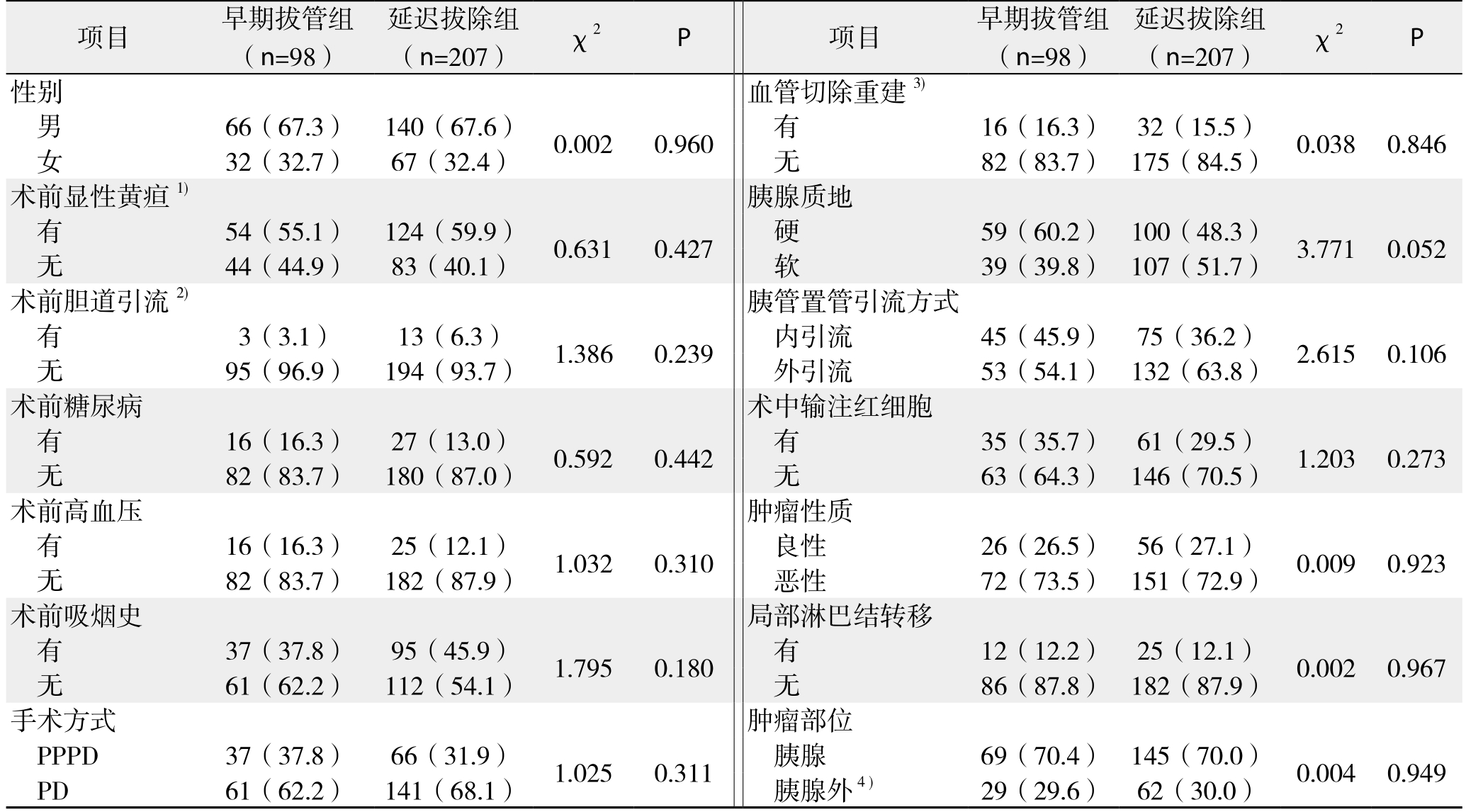
注:1)显性黄疸指血清TBIL>34.2 μmol/L;2)胆道引流包括PTCD、ERCP放置胆道内支架或鼻胆管3种方式;3)血管切除重建包括门静脉、肝动脉切除重建两种方式;4)胰腺外肿瘤包括胆总管下段、十二指肠乳头部、胆胰壶腹部的肿瘤
Note: 1) Dominant jaundice referring to 34.2 μmol/L; 2) Biliary drainage including PTCD, and biliary stent or nasobiliary tube placement through ERCP; 3) Vascular resection and reconstruction including resection and reconstruction of the portal vein and hepatic artery;4) Extrapancreatic tumors referring the tumors in the distal common bile duct, duodend papilla and the ampulla of Vater
项目 早期拔管组(n=98)延迟拔除组(n=207) χ2 P 项目 早期拔管组(n=98)延迟拔除组(n=207) χ2 P性别 血管切除重建3)男66(67.3) 140(67.6) 0.002 0.960 有 16(16.3) 32(15.5) 0.038 0.846女32(32.7) 67(32.4) 无 82(83.7) 175(84.5)术前显性黄疸1) 胰腺质地有54(55.1) 124(59.9) 0.631 0.427 硬 59(60.2) 100(48.3) 3.771 0.052无44(44.9) 83(40.1) 软 39(39.8) 107(51.7)术前胆道引流2) 胰管置管引流方式有3(3.1) 13(6.3) 1.386 0.239 内引流 45(45.9) 75(36.2) 2.615 0.106无95(96.9) 194(93.7) 外引流 53(54.1) 132(63.8)术前糖尿病 术中输注红细胞有16(16.3) 27(13.0) 0.592 0.442 有 35(35.7) 61(29.5) 1.203 0.273无82(83.7) 180(87.0) 无 63(64.3) 146(70.5)术前高血压 肿瘤性质有16(16.3) 25(12.1) 1.032 0.310 良性 26(26.5) 56(27.1) 0.009 0.923无82(83.7) 182(87.9) 恶性 72(73.5) 151(72.9)术前吸烟史 局部淋巴结转移有37(37.8) 95(45.9) 1.795 0.180 有 12(12.2) 25(12.1) 0.002 0.967无61(62.2) 112(54.1) 无 86(87.8) 182(87.9)手术方式 肿瘤部位PPPD 37(37.8) 66(31.9) 1.025 0.311 胰腺 69(70.4) 145(70.0) 0.004 0.949 PD 61(62.2) 141(68.1) 胰腺外4) 29(29.6) 62(30.0)
表2 早期拔管组与延迟拔除组患者基线资料(计量资料)比较[M(范围)]
Table 2 Comparison of baseline data (measurement data) between early tube removal group and delayed tube removal group [M(range)]
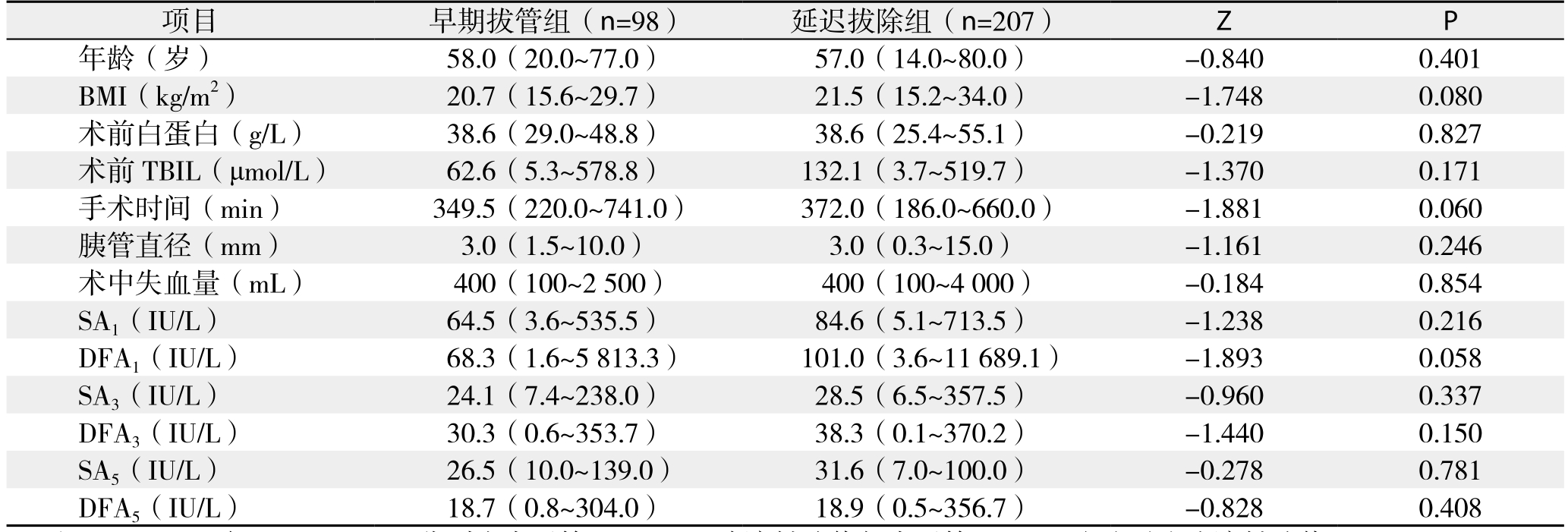
注:SA3、SA5与DFA3、DFA5分别为术后第3、5天血清淀粉酶值与术后第3、5天腹腔引流液淀粉酶值
Note: SA3, SA5, and DFA3, DFA5 referring to the drainfluid amylase and serum amylase on POD 3 and 5
项目 早期拔管组(n=98) 延迟拔除组(n=207) Z P年龄(岁) 58.0(20.0~77.0) 57.0(14.0~80.0) -0.840 0.401 BMI(kg/m2) 20.7(15.6~29.7) 21.5(15.2~34.0) -1.748 0.080术前白蛋白(g/L) 38.6(29.0~48.8) 38.6(25.4~55.1) -0.219 0.827术前TBIL(μmol/L) 62.6(5.3~578.8) 132.1(3.7~519.7) -1.370 0.171手术时间(min) 349.5(220.0~741.0) 372.0(186.0~660.0) -1.881 0.060胰管直径(mm) 3.0(1.5~10.0) 3.0(0.3~15.0) -1.161 0.246术中失血量(mL) 400(100~2 500) 400(100~4 000) -0.184 0.854 SA1(IU/L) 64.5(3.6~535.5) 84.6(5.1~713.5) -1.238 0.216 DFA1(IU/L) 68.3(1.6~5 813.3) 101.0(3.6~11 689.1) -1.893 0.058 SA3(IU/L) 24.1(7.4~238.0) 28.5(6.5~357.5) -0.960 0.337 DFA3(IU/L) 30.3(0.6~353.7) 38.3(0.1~370.2) -1.440 0.150 SA5(IU/L) 26.5(10.0~139.0) 31.6(7.0~100.0) -0.278 0.781 DFA5(IU/L) 18.7(0.8~304.0) 18.9(0.5~356.7) -0.828 0.408
表3 早期拔管组与延迟拔除组患者术后并发症比较
Table 3 Comparison of postoperative complications between early tube removal group and delayed tube removal group
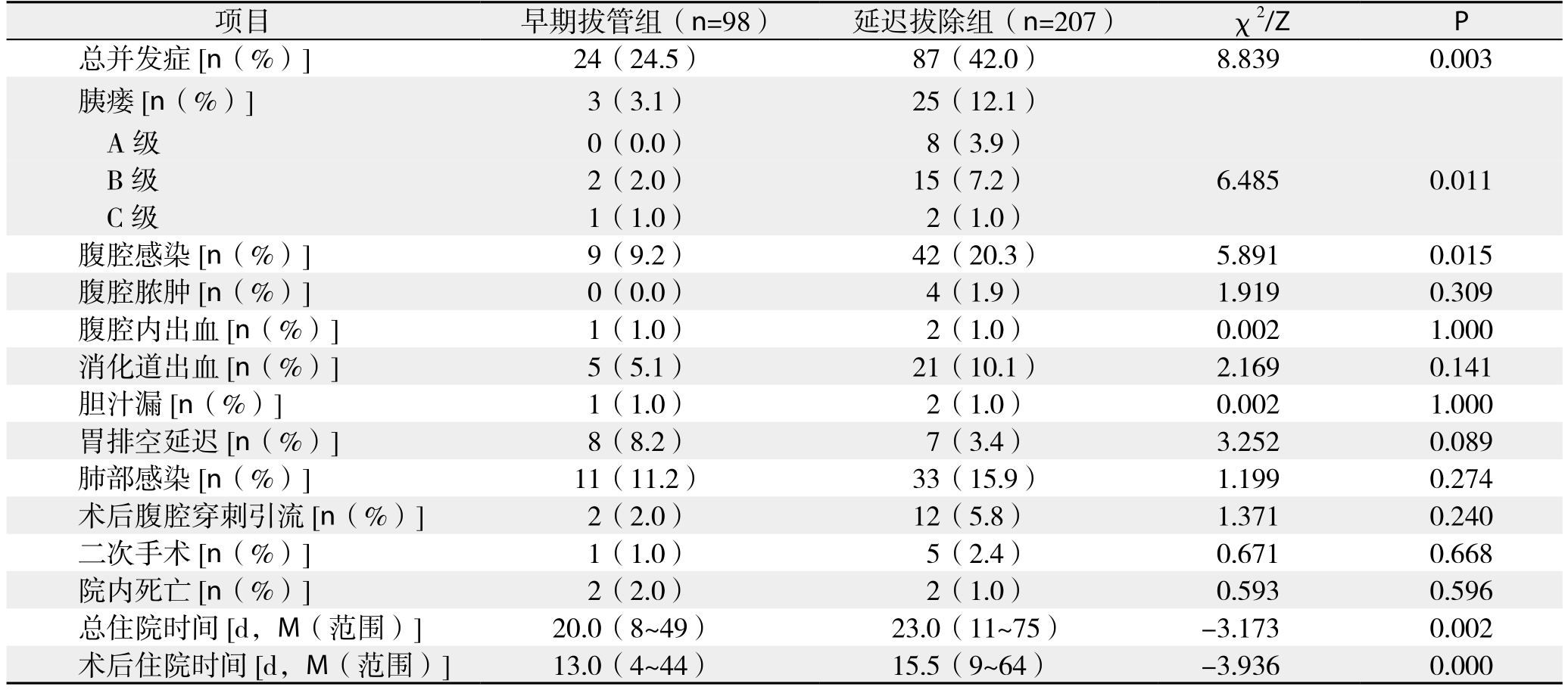
项目 早期拔管组(n=98) 延迟拔除组(n=207) χ2/Z P总并发症[n(%)] 24(24.5) 87(42.0) 8.839 0.003胰瘘 [n(%)] 3(3.1) 25(12.1)A级 0(0.0) 8(3.9)B级 2(2.0) 15(7.2) 6.485 0.011 C级 1(1.0) 2(1.0)腹腔感染[n(%)] 9(9.2) 42(20.3) 5.891 0.015腹腔脓肿[n(%)] 0(0.0) 4(1.9) 1.919 0.309腹腔内出血[n(%)] 1(1.0) 2(1.0) 0.002 1.000消化道出血[n(%)] 5(5.1) 21(10.1) 2.169 0.141胆汁漏[n(%)] 1(1.0) 2(1.0) 0.002 1.000胃排空延迟[n(%)] 8(8.2) 7(3.4) 3.252 0.089肺部感染[n(%)] 11(11.2) 33(15.9) 1.199 0.274术后腹腔穿刺引流[n(%)] 2(2.0) 12(5.8) 1.371 0.240二次手术[n(%)] 1(1.0) 5(2.4) 0.671 0.668院内死亡[n(%)] 2(2.0) 2(1.0) 0.593 0.596总住院时间[d,M(范围)] 20.0(8~49) 23.0(11~75) -3.173 0.002术后住院时间[d,M(范围)] 13.0(4~44) 15.5(9~64) -3.936 0.000
表4 PD术后早期胰瘘发生的单因素分析(计数资料)
Table 4 Univariate analysis of early POPF following PD (enumeration data)
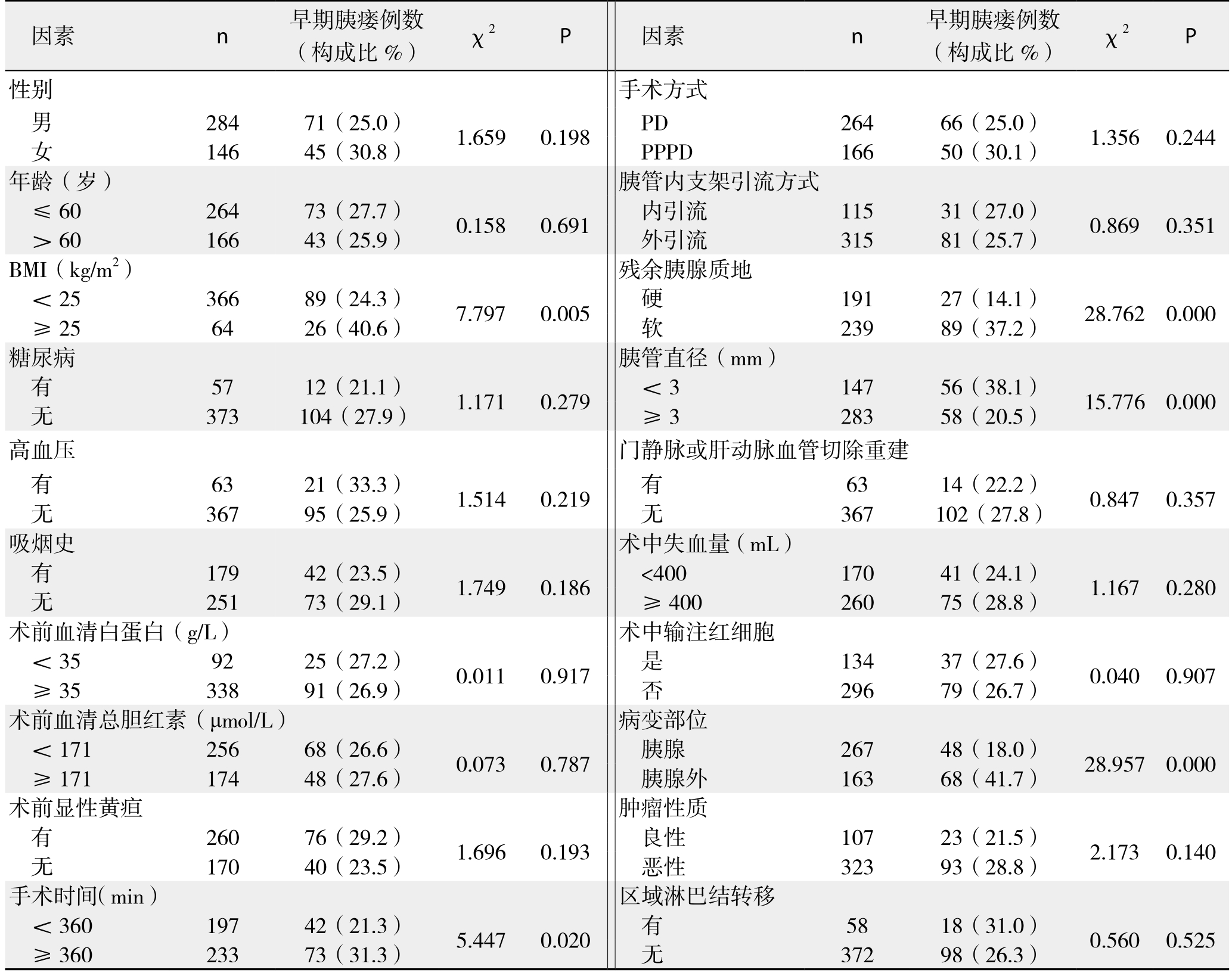
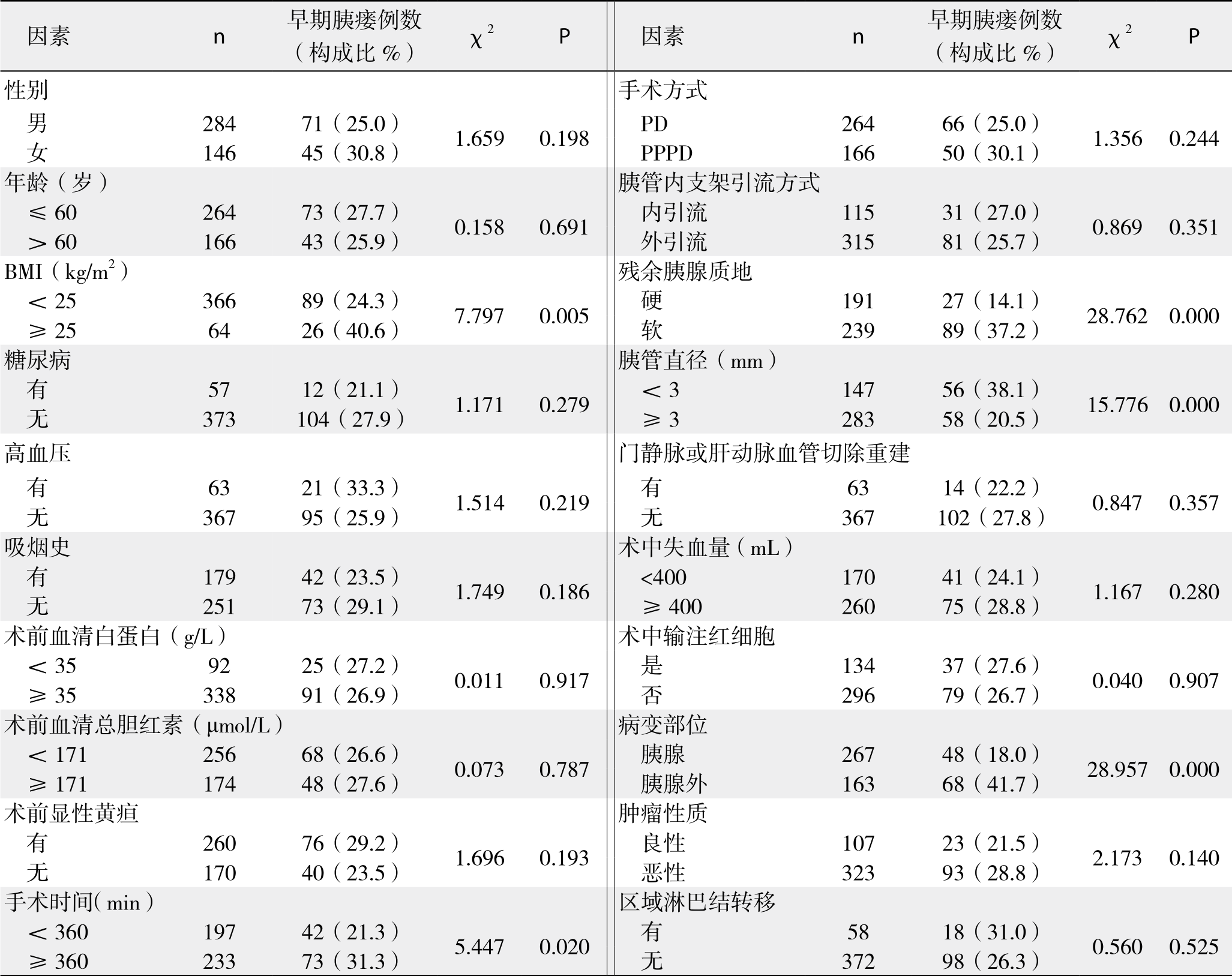
表5 PD术后早期胰瘘发生的单因素分析结果(计量资料)
Table 5 Univariate analysis of early POPF following PD (measurement data)
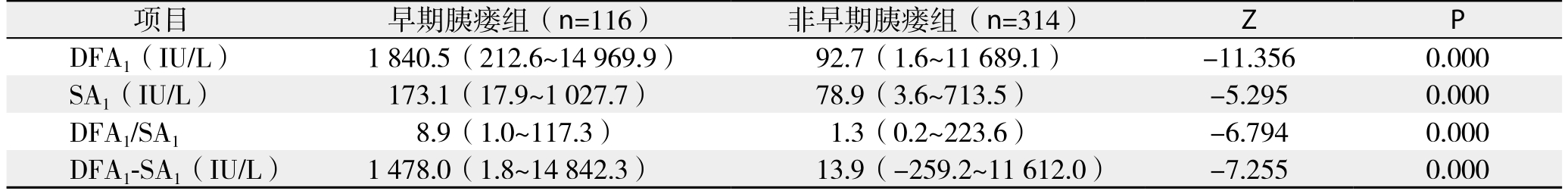
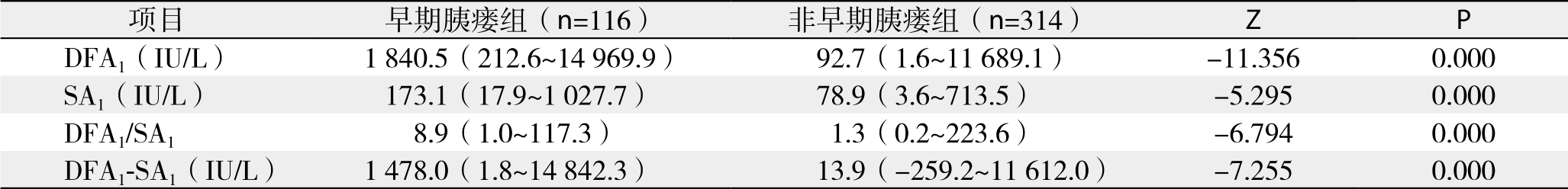
表6 PD术后早期胰瘘发生的多因素Logistic回归分析结果
Table 6 Results of multivariate logistic regression analysis for early POPF following PD
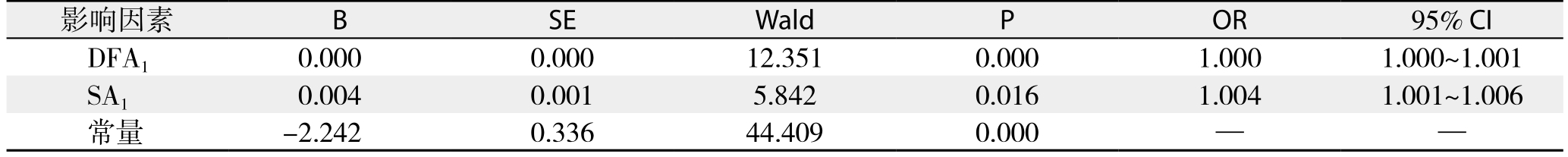

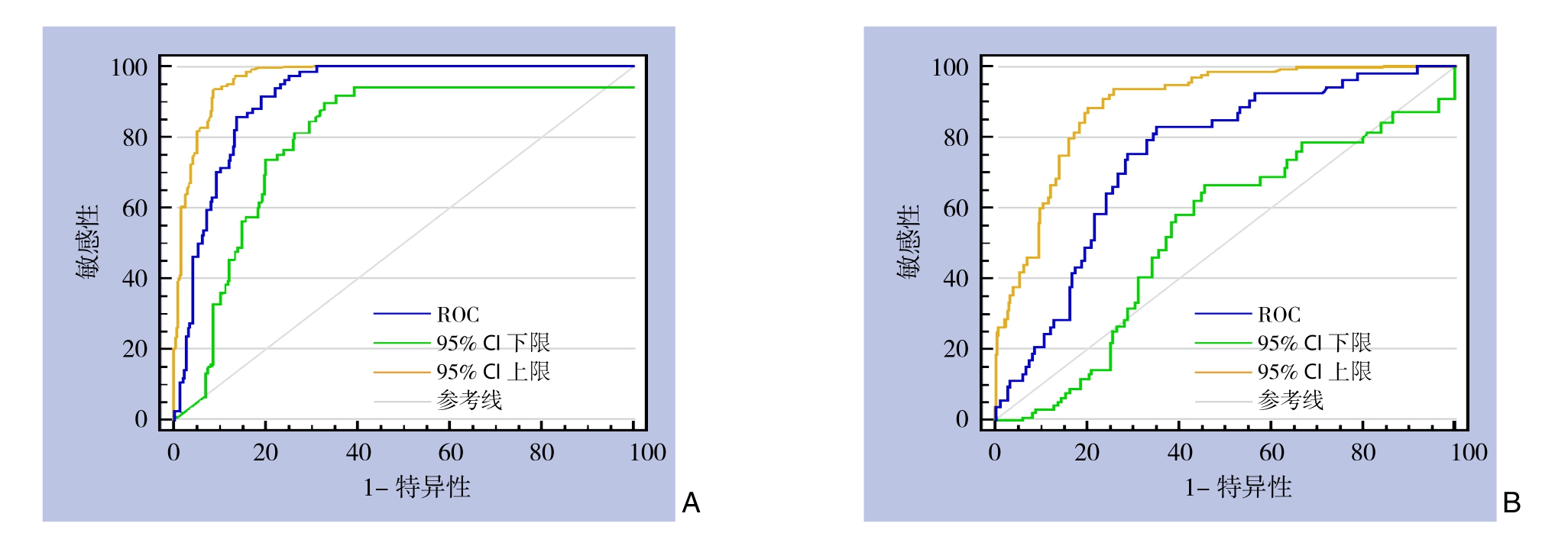
图1 预测PD术后早期胰瘘发生ROC曲线 A:DFA1(AUC=0.916,P<0.001,95% CI=88.7%~94.6%);B:SA1(AUC=0.745,P<0.001,95% CI=67.3%~81.8%)
Figure 1 ROC curves of prediction of early POPF after PD A: DFA1 (AUC=0.916, P<0.001, 95% CI=88.7%–94.6%); B: SA1 (AUC=0.745,P<0.001, 95% CI=67.3%–81.8%)
表7 DFA1与SA1预测PD术后早期胰瘘发生的截断值
Table 7 Cut-off values of DFA1 and SA1 for predicting early POPF after PD

项目 AUC 截断值(IU/L) 敏感度 特异度 阳性预测值(95% CI) 阴性预测值(95% CI)DFA1 0.916 494.75 91.7 80.8 62.7(53.5~71.2) 96.5(92.9~98.6)SA1 0.745 96.35 83.0 64.9 45.9(35.7~56.4) 91.4(84.3~96.0)
3 讨 论
胰瘘为PD术后最常见、也最严重的并发症,文献[11,22]报道发生率从2%~46%不等,且发生后容易引起腹腔出血、腹腔脓肿、器官功能衰竭等一系列问题,威胁患者生命[21-23]。多年来外科医生尝试采取多种措施来减少术后胰瘘的发生,包括预防性使用奥曲肽等生长抑素类似物[24-26],尝试胰胃吻合或胰腺空肠套入式吻合等手术方式[27-28],以及术中放置胰管内支架行胰液引流[29-30]等,但均收效甚微。后来Kawai等[19]和Bassi等[31]在研究中发现,术后早期拔除腹腔引流管能够显著降低术后胰瘘、腹腔感染以及肺部感染等并发症的发生率。在本次研究中我们也进行了验证分析,结果得出,对于PD术后无早期胰瘘发生的患者,腹腔引流管术后5 d内拔除相对5 d以后拔除显著降低了术后胰瘘和腹腔感染的发生几率,且不会增加术后腹腔穿刺引流和二次手术的概率,在此基础上,笔者继续对PD术后早期胰瘘发生的影响因素进行分析,得出DFA1、SA1为术后早期胰瘘的独立影响因素,并利用它们进一步对早期胰瘘进行预测,最终确定对于DFA1<494.75 IU/L的患者(敏感度、特异度、阳性预测值、阴性预测值分别为91.7%、80.8%、62.7%、96.5%),可于PD术后第3天安全拔除腹腔引流管,并可对患者实施快速康复策略。
PD术后早期拔除腹腔引流管减少了术后胰瘘等并发症的发生,笔者分析可能有以下几方面的原因。一是术后使用负压吸引虽能有效引流腹腔内积液、积血,但若长时间留置其强大的负压吸引力也能侵蚀、破坏邻近组织特别是吻合口,影响其愈合;二是腹腔引流管无形之中可能成为了细菌进入腹腔的通道,增加了吻合口周围感染的几率;三是术后胰瘘与腹腔感染这两个因素可能相互促进,胰瘘能激活人体免疫系统,导致全身炎症反应发生,这一过程可能会引起肠道细菌移位,造成腹腔感染,而细菌含有的磷脂酶和脂多糖反过来也能激活漏出的胰液[32],促进胰瘘的进一步发展,从而造成恶性循环,影响患者术后康复。
本次研究发现,对于PD术后无早期胰瘘发生的患者,长期使用腹腔引流明显增加了术后胰瘘、腹腔感染等的发生,且早期拔除腹腔引流管的98例患者中,只有3例(3.1%)发生了术后胰瘘。为更准确预测术后胰瘘,尽量减轻腹腔引流管对其产生的影响,我们采取对术后早期胰瘘的发生风险进行预测分析,结果显示,DFA1对术后早期胰瘘有较好的预测价值(AUC=0.916,P<0.001,95% CI=88.7%~94.6%)。另外,本次研究中术后第5天这一时间分界点参考了国内外一些大型胰腺外科中心的胰腺切除术后标准管理策略,他们将PD术后第5天作为没有出现临床可疑胰瘘患者的常规腹腔引流管拔除时间[31]。
近年来,国内外关于DFA1预测胰腺切除术后胰瘘发生的报道屡见不鲜。Molinari等[6]较早在研究中得出,胰腺切除术后DFA1≥5 000 U/L是预测术后胰瘘发生的唯一指标,后来Partelli等[33]和Nissen等[34]也在各自的研究中得出了相似的结论。本研究中,我们也尝试利用DFA1≥5 000 U/L对PD术后胰瘘进行预测分析,结果得出其敏感度、特异度、阳性预测值、阴性预测值分别为16.5%、98.1%、81.5%和69.8%,预测价值不高的原因可能在于Molinari和Partelli的研究中不仅包含着PD手术,也包含着胰体尾切除术,而Nissen等[34]的研究中虽只包括PD手术患者,但病例样本量却较少。之后Kawai等[12]回顾分析了1 239例行PD手术患者的临床资料,得出DFA1>4 000 IU/L预测术后临床胰瘘(B、C级胰瘘)的敏感度、特异度、准确度分别为62.2%、89.0%、84.8%;Sutcliffe等[7]则在一项前瞻性研究中分析70例行PD手术患者的临床资料后得出,DFA1≤350 U/L排除术后胰瘘的敏感度、特异度、阳性预测值、阴性预测值分别为100%、79%、41%和100%;Israel等[8]则利用前瞻性数据库分析63例记录有DFA1值患者的临床资料,总结出DFA1>100U/L预测胰腺切除术后胰瘘发生有较高的敏感度和阴性预测值(均为96%);Lee等[11]回顾分析数据库中536例记录有DFA1的胰腺切除手术患者病例资料,提出DFA1<90U/L排除术后胰瘘的阴性预测值最高(98.2%)。虽然相关研究报道较多,但DFA1预测术后胰瘘发生的截断值跨度较大,且多数研究既包含PD手术,也包含胰体尾切除术,临床应用价值有限;而本次研究对象全部为PD手术患者,并均采用胰腺导管对空场黏膜端侧吻合方式重建消化道,所得结论对指导患者术后康复更有临床意义。
此外,目前有部分研究[35-36]报道SA1对PD术后胰瘘发生也具有较好的预测价值。受此启发,我们将DFA1、SA1、两者比值、两者差值均纳入对术后早期胰瘘的预测分析中,结果显示只有DFA1和SA1为PD术后早期胰瘘的独立影响因素,而其中DFA1预测价值更高(AUC:0.916 vs. 0.745)。最后通过计算确定DFA1≥494.75 IU/L为预测PD术后早期胰瘘发生的唯一条件(敏感度、特异度、阳性预测值、阴性预测值分别为91.7%、80.8%、62.7%、96.5%)。
本次研究为回顾性病例分析研究,论证级别相对较低,且个别患者术后并未记录DFA1、SA1等值,使研究结果受到一定影响,未来应设计前瞻性临床研究对该结论予以进一步验证,以更准确地指导临床应用。
综上所述,对于PD术后无早期胰瘘发生的患者,术后5 d内拔除腹腔引流管能降低术后胰瘘和腹腔感染的发生率;且对于DFA1<494.75 IU/L的患者,可于术后3天安全拔除腹腔引流管,并对患者实施快速康复策略。
参考文献
[1]El Nakeeb A, Salah T, Sultan A, et al. Pancreatic anastomotic leakage after pancreaticoduodenectomy. Risk factors, clinical predictors, and management (single center experience)[J]. World J Surg, 2013, 37(6):1405–1418. doi: 10.1007/s00268–013–1998–5.
[2]Whipple AO. Pancreaticoduodenectomy for Islet Carcinoma: A Five-Year Follow-Up[J]. Ann Surg, 1945, 121(6):847–852.
[3]Schnelldorfer T, Ware AL, Sarr MG, et al. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible?[J]. Ann Surg, 2008, 247(3):456–462. doi: 10.1097/SLA.0b013e3181613142.
[4]Peng L, Lin S, Li Y, et al. Systematic review and meta-analysis of robotic versus open pancreaticoduodenectomy[J]. Surg Endosc,2017, 31(8):3085–3097. doi: 10.1007/s00464–016–5371–2.
[5]Chen JY, Feng J, Wang XQ, et al. Risk scoring system and predictor for clinically relevant pancreatic fistula after pancreaticoduodenectomy[J]. World J Gastroenterol, 2015,21(19):5926–5933. doi: 10.3748/wjg.v21.i19.5926.
[6]Molinari E, Bassi C, Salvia R, et al. Amylase value in drains after pancreatic resection as predictive factor of postoperative pancreatic fistula: results of a prospective study in 137 patients[J]. Ann Surg,2007, 246(2):281–287.
[7]Sutcliffe R P, Battula N, Haque A, et al. Utility of drain fluid amylase measurement on the first postoperative day after pancreaticoduodenectomy[J]. World J Surg, 2012, 36(4):879–883.doi: 10.1007/s00268–012–1460–0..
[8]Israel JS, Rettammel RJ, Leverson GE, et al. Does postoperative drain amylase predict pancreatic fistula after pancreatectomy?[J]. J Am Coll Surg, 2014, 218(5):978–987. doi: 10.1016/j.jamcollsurg.2014.01.048.
[9]Dai J, Jiang Y, Fu D. Reducing postoperative complications and improving clinical outcome: Enhanced recovery after surgery in pancreaticoduodenectomy-Aretrospective cohort study[J]. Int J Surg, 2017, 39:176–181. doi: 10.1016/j.ijsu.2017.01.089.
[10]Shah OJ, Bangri SA, Singh M, et al. Impact of centralization of pancreaticoduodenectomy coupled with fast track recovery protocol: a comparative study from India[J]. Hepatobiliary Pancreat Dis Int, 2016, 15(5):546–552.
[11]Lee CW, Pitt HA, Riall TS, et al. Low drainfluid amylase predicts absence of pancreatic fistula following pancreatectomy[J]. J Gastrointest Surg, 2014, 18(11):1902–1910. doi: 10.1007/s11605–014–2601–6.
[12]Kawai M, Kondo S, Yamaue H, et al. Predictive risk factors for clinically relevant pancreatic fistula analyzed in 1,239 patients with pancreaticoduodenectomy: multicenter data collection as a project study of pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery [J]. J Hepatobiliary Pancreat Sci, 2011,18(4):601–608. doi: 10.1007/s00534–011–0373–x.
[13]Tandon P, Reddy K R, O'Leary J G, et al. A Karnofsky performance status-based score predicts death after hospital discharge in patients with cirrhosis [J]. Hepatology, 2017, 65(1):217–224. doi: 10.1002/hep.28900.
[14]Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition[J]. Surgery,2005, 138(1):8–13.
[15]朱宏达, 马尘超, 白岳青, 等. 胰十二指肠切除术后腹腔并发症发生及引流留置时间预测[J]. 肝胆胰外科杂志, 2014, 26(5):372–375.Zhu HD, Ma CC, Bai YQ, et al. Prediction for abdominal complications and drainage indwelling duration after pancreaticoduodenectomy[J]. Journal of Hepatopancreatobiliary Surgery, 2014, 26(5):372–375.
[16]马炳奇,张顺,张斌,等. 胰十二指肠切除术后胰瘘发生的相关性因素分析[J].中国普通外科杂志,2013,22:(3): 315–319.doi:10.7659/j.issn.1005–6947.2013.03.011.Ma BQ, Zhang S, Zhang B,et al. An analysis of factors responsible for pancreatic fistula after pancreaticoduodenectomy[J]. Chinese Journal of General Surgery,2013,22:(3): 315–319. doi:10.7659/j.issn.1005–6947.2013.03.011.
[17]Wente M N, Veit J A, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition[J]. Surgery, 2007, 142(1):20–25.
[18]Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery[J].Surgery, 2011, 149(5):680–688. doi: 10.1016/j.surg.2010.12.002.
[19]Kawai M, Tani M, Terasawa H, et al. Early removal of prophylactic drains reduces the risk of intra-abdominal infections in patients with pancreatic head resection: prospective study for 104 consecutive patients[J]. Ann Surg, 2006, 244(1):1–7.
[20]Wente M N, Bassi C, Dervenis C, et al. Delayed gastric emptying(DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS)[J].Surgery, 2007, 142(5):761–768.
[21]Meng G, Xing Q, Yuan Q, et al. Internal compared with external drainage of pancreatic duct during pancreaticoduodenectomy: a retrospective study[J]. Chin J Cancer Res, 2014, 26(3):277–284.doi: 10.3978/j.issn.1000–9604.2014.06.05.
[22]Yang J, Huang Q, Wang C. Postoperative drain amylase predicts pancreatic fistula in pancreatic surgery: A systematic review and meta-analysis [J]. Int J Surg, 2015, 22:38–45. doi: 10.1016/j.ijsu.2015.07.007.
[23]Wang YC, Szatmary P, Zhu J Q, et al. Prophylactic intra-peritoneal drain placement following pancreaticoduodenectomy: A systematic review and meta-analysis[J]. World J Gastroenterol, 2015,21(8):2510–2521. doi: 10.3748/wjg.v21.i8.2510.
[24]Jin K, Zhou H, Zhang J, et al. Systematic review and meta-analysis of somatostatin analogues in the prevention of postoperative complication after pancreaticoduodenectomy[J]. Dig Surg, 2015,32(3):196–207.
[25]Kurumboor P, Palaniswami K N, Pramil K, et al. Octreotide Does Not Prevent Pancreatic Fistula Following Pancreatoduodenectomy in Patients with Soft Pancreas and Non-dilated Duct: A Prospective Randomized Controlled Trial[J]. J Gastrointest Surg, 2015,19(11):2038–2044. doi: 10.1007/s11605–015–2925–x.
[26]孔瑞, 胡继盛, 李乐, 等. 奥曲肽对胰十二指肠切除术后胰瘘影响的前瞻性研究[J]. 中华外科杂志, 2016, 54(1):21–24. doi:10.3760/cma.j.issn.0529–5815.2016.01.006.Kong R, Hu JS, Li L, et al. Impact of octreotide on pancreatic fistula after pancreaticoduodenectomy: a prospective study[J].Chinese Journal of Surgery, 2016, 54(1):21–24. doi: 10.3760/cma.j.issn.0529–5815.2016.01.006.
[27]El Nakeeb A, El Hemaly M, Askr W, et al. Comparative study between duct to mucosa and invagination pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized study[J].Int J Surg, 2015, 16(Pt A):1–6. doi: 10.1016/j.ijsu.2015.02.002.
[28]Guerrini GP, Soliani P, D'Amico G, et al. Pancreaticojejunostomy Versus Pancreaticogastrostomy After Pancreaticoduodenectomy: An Up-to-date Meta-Analysis[J]. J Invest Surg, 2016, 29(3):175–184.doi: 10.3109/08941939.2015.1093047.
[29]Usuba T, Misawa T, Ito R, et al. Safety of Non-stented Pancreaticojejunostomy in Pancreaticoduodenectomy for Patients with Soft Pancreas[J]. Anticancer Res, 2016, 36(12):6619–6623.
[30]Ke FY, Wu XS, Zhang Y, et al. Comparison of postoperative complications between internal and external pancreatic duct stenting during pancreaticoduodenectomy: a meta-analysis[J]. Chin J Cancer Res, 2015, 27(4):397–407. doi: 10.3978/j.issn.1000–9604.2015.07.04.
[31]Bassi C, Molinari E, Malleo G, et al. Early versus late drain removal after standard pancreatic resections: results of a prospective randomized trial[J]. Ann Surg, 2010, 252(2):207–214. doi: 10.1097/SLA.0b013e3181e61e88.
[32]Vaccaro MI, Dagrosa MA, Mora MI, et al. The effect of chronic intraperitoneal infusion of bacterial endotoxin on exocrine pancreas function in rats[J]. Int J Pancreatol, 1996, 19(1):49–54.
[33]Partelli S, Tamburrino D, Crippa S, et al. Evaluation of a predictive model for pancreatic fistula based on amylase value in drains after pancreatic resection[J]. Am J Surg, 2014, 208(4):634–639. doi:10.1016/j.amjsurg.2014.03.011.
[34]Nissen NN, Menon VG, Puri V, et al. A simple algorithm for drain management after pancreaticoduodenectomy[J]. Am Surg, 2012,78(10):1143–1146.
[35]Cloyd J M, Kastenberg Z J, Visser B C, et al. Postoperative serum amylase predicts pancreatic fistula formation following pancreaticoduodenectomy[J]. J Gastrointest Surg, 2014, 18(2):348–353. doi: 10.1007/s11605–013–2293–3.
[36]Kühlbrey CM, Samiei N, Sick O, et al. Pancreatitis After Pancreatoduodenectomy Predicts Clinically Relevant Postoperative Pancreatic Fistula[J]. J Gastrointest Surg, 2017, 21(2):330–338. doi:10.1007/s11605–016–3305–x.