胰腺良性疾病发病率高于恶性疾病,以囊性疾病、神经内分泌肿瘤和实性假乳头状肿瘤最为常见[1-3],多数需手术治疗,理想的手术方式是剜除或局部切除。当病变体积稍大、与主胰管稍近(<2~3 mm安全距离)[3-4],或术中损伤主胰管时,众多国内外新旧指南[5-9]均推荐节段性切除、胰肠吻合或胰十二指肠切除术。这些手术方法对于胰腺良性疾病而言,目的性创伤过大,牺牲过多胰腺组织,改变正常生理解剖。
传统观念认为主胰管无法修复、无法重建和无法替代,因此只能通过扩大切除或消化道重建去处理主胰管缺损,笔者对此一直存有质疑,胰腺、空肠、胃壁可以吻合,为什么胰腺与胰腺、胰管与胰管不能重建呢?受一个偶然的成功病例启发,笔者创新采用桥梁对拢理论进行胰腺良性疾病新术式的临床探索性研究,手术方式主要以主胰管架桥修复[10]和选择胰腺端端对吻重建术[11]为基础,对良性疾病切除后缺损的胰腺进行整形修复术,还原正常解剖,恢复主胰管连续性,初步临床结果较好地证实了该理论和技术方法的可行性和安全性,有望彻底改变胰腺良性疾病的外科治疗的方法。
1 临床探索实践和疗效
2016年10月—2018年1月间中国人民解放军总医院共收治17例胰腺单发占位性病变,男:女=8:9,年龄22~62岁,影像学检查提示肿瘤均紧靠或推移主胰管(图1),术后病理提示实性假乳头状肿瘤7例,黏液性囊腺瘤1例,浆液性囊腺瘤6例,慢性肿块性胰腺炎1例,2例肾透明细胞癌术后胰腺转移,病变直径0.7~4.1 cm,17例患者中4例采用剜除术切除肿瘤,13例采用中段胰腺切除移除肿瘤,主胰管术中缺损0~6 cm(主胰管仅前壁损伤、后壁完整的病例,损伤距离计0 cm),笔者创新性应用桥梁对拢修复理论对主胰管和胰腺实质进行整形修复,其中剜除术后2例主胰管架桥修复,2例主胰管架桥修复+胰管包埋,中段胰腺切除术后11例主胰管架桥修复+胰腺端端对吻重建,2例主胰管架桥修复+胰管旷置,所有患者术后均发生胰瘘,多为BL和B级胰瘘[12],无C级胰瘘发生,术后13 d至10个月均顺利拔除腹腔引流管,无1例形成胰管皮肤瘘,随访1~16个月,影像学检查提示3例主胰管架桥修复+胰管旷置术后患者胰腺支撑管未脱落,其余胰腺支撑管均自行脱落。

图1 胰腺肿瘤与主胰管关系密切
Figure 1 Pancreatic tumor with close relation to the main pancreatic duct
2 具体手术方法介绍及注意事项
所有手术均采用达芬奇机器人手术系统(Si-HD)完成。患者取平卧位,参考胰十二指肠切除术或远端胰腺切除术布孔,5孔法操作。首先超声刀大范围打开胃结肠韧带,显露胰腺体部,打开胰腺下缘结肠系膜前叶,显露肠系膜上静脉,选择性打通胰头隧道,将肿瘤两侧胰腺完整游离,根据胰腺病变的大小,采用剜除或节段性切除方法切除肿瘤,其后根据主胰管损伤长度和周围胰腺残留情况,采用3种方法对胰腺进行一期整形修复术:⑴ 主胰管架桥修复+选择性胰腺支撑管包埋术(图2),适用于剜除术后主管损伤或缺损≤3 cm的病例。术中找到主胰管两侧断端,选择适宜直径的胰腺支撑管分别放入主胰管近端和远端,使用5-0可吸收线将胰管和主胰管固定,外源性胰腺支撑管取代主胰管,修复主胰管的连续性,根据胰腺创面情况选择性对拢缝合胰腺创面,包埋胰管。⑵ 主胰管架桥修复+胰腺端端对吻重建术(图3),适用于胰腺中段切除术后主胰管缺损≤5 cm。术中同样选择适宜直径胰腺支撑管放入主胰管,修复主胰管连续性,远端5-0可吸收线固定,近端不固定,胰腺两端断面上下缘4-0 Prolene垂直八字缝合,中间U型缝合,待两侧胰腺创面处理完毕后,使用4-0或3-0 Prolene间断或连续缝合将胰腺端端对吻,胰腺支撑管内置。⑶ 主胰管架桥修复+胰管旷置术(图4),适用于中段胰腺切除术后主胰管缺损>5 cm。术中选择适宜直径胰腺支撑管放入主胰管,修复主胰管的连续性,远端和近端均采用可吸收线固定,两侧胰腺断端U型缝合,闭合断面小的胰管断端。

图2 肿瘤剜除术后根据主胰管缺损情况行主胰管架桥修复术(主胰管缺损≤3 cm,左),选择性胰腺支撑管包埋术(主胰管缺损3~5 cm,右)
Figure 2 According to the defect dimension after tumor excision, performing main pancreatic duct bridging repair (defect of the main pancreatic duct ≤3 cm, left), and selective pancreatic stent embedment (defect of the main pancreatic duct within 3–5 cm,right)

图3 中段胰腺切除术后主胰管缺损≤5 cm,行主胰管架桥修复+胰腺端端对吻重建术
Figure 3 Bridging repair of the main pancreatic duct plus end-to-end pancreatic anastomosis reconstruction for defect of the main pancreatic duct ≤5 cm after central pancreatectomy
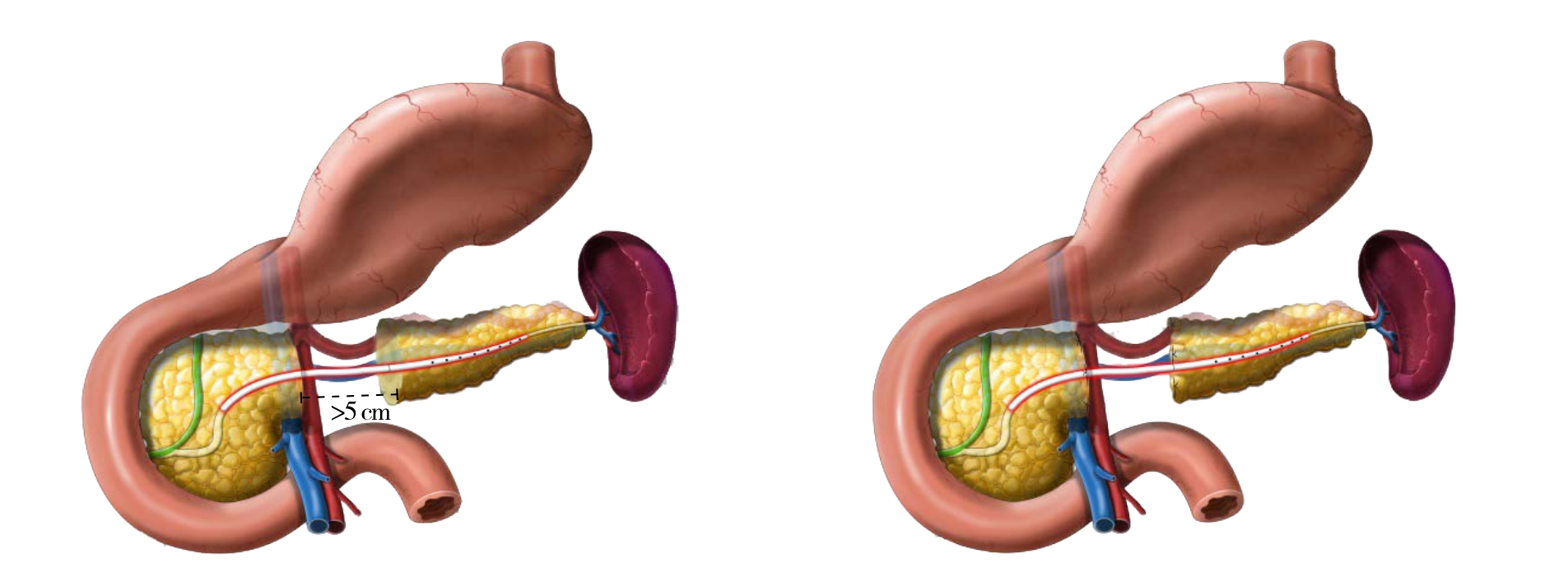
图4 中段胰腺切除术后主胰管缺损>5 cm,行主胰管架桥修复+胰腺支撑管旷置术
Figure 4 Bridging repair of the main pancreatic duct pluspancreatic stent exclusion for defect of the main pancreatic duct >5 cm after central pancreatectomy
手术操作要点:⑴ 胰管多可自行脱落,因此胰管应选用可吸收线固定为主;⑵ 胰管固定时,远端主胰管与胰管应固定严密,减少主胰管与胰管间胰液外漏的可能,胰管附近U型缝合不失为一种好方法;⑶ 剜除术后主胰管缺损>3 cm病例,应采用中段胰腺切除,选择胰腺端端对吻重建或主胰管旷置术;⑷ 胰腺端端对吻重建时,5 cm以下距离对拢拉合一般没有问题,前提做到两端充分游离,以远端胰腺游离为主;⑸ 术后引流应充分,常规建议2根粗乳胶,分别置于胰腺上缘和下缘;⑹ 术后建议常规给予生长抑素,临床观察提示可显著减少胰瘘引流量,减少胰管皮肤瘘的发生几率。
3 桥梁合拢理论与技术的介绍
新术式中主要内容与工程建筑中的桥梁建造过程相近,因此笔者用桥梁合拢理论来描述该技术方法。胰腺质地松软,单纯胰端端拉合过程中极易切割胰断端,笔者首先采用上下垂直8字、中间U型缝合的方法处理胰腺断端,止血、缝闭胰腺断端小的分支胰管同时,为后续胰腺拉合做好“桥桩”,再以“桥桩”为基点将胰端端缝合、拉拢,如两端距离较长,可分次拉合。两侧游离好的胰腺断端,有如的桥梁两端,最后在门静脉上方形成桥梁的合拢,主胰管犹如桥梁表面的交通道路,因此桥梁合拢后也需形成道路,胰腺端端对吻也需恢复主胰管连续性。剜除术后相当于道路受损,主要修复“道路”即可,主胰架桥修复即可;中段切除术后修复“道路”的同时还应合拢“桥梁”,当”桥梁”缺损过长时无法合拢,只能将桥梁两端封死,中间做简易架桥,连接“桥梁”两端。新术式3种方法均可用桥梁合拢理论作为基础来类比。
4 未知问题的探讨
近20例成功的病例让笔者看到了新术式的巨大潜力,但也引来较多思考。如术后如何生长抑素使用规范、主胰管缺损段胰腺组织外分泌功能如何实现、长期外源性胰管内置的远期并发症、胰腺支撑管旷置后胰腺能否再生。此外,临床手术适应证还需明确等等,众多问题有待进一步基础和临床研究论证。
5 新术式的价值和发展潜力
随着经验的积累和技术的进步,目前胰腺良性疾病几乎均采用微创方法进行[13-21],机器人或腹腔镜手术,但前述微创手术均减少的是手术入路创伤,胰腺肿瘤切除的手术方式一直没有变化,即目的性创伤没有得到任何的减少,笔者借助桥梁合拢理论应用主胰管架桥修复和胰腺端端对吻重建术改变了胰腺良性疾病传统外科方法,该理论适用于近乎所有胰腺良性疾病。胰腺良性疾病可以采用目的性创伤最小的剜除或节段性切除方法去除,其后进行主胰管和胰腺实质整形修复,最大程度的保留正常胰腺组织,保证生理解剖的完整性,初步临床疗效极佳,推荐同行们推广应用。
参考文献
[1]Halfdanarson TR, Rubin J, Farnell MB, et al. Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumors[J]. Endocr Relat Cancer, 2008, 15(2):409–427. doi: 10.1677/ERC-07–0221.
[2]Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas[J]. Pancreatology, 2012, 12(3):183–197. doi:10.1016/j.pan.2012.04.004.
[3]Heeger K, Falconi M, Partelli S, et al. Increased rate of clinically relevant pancreatic fistula after deep enucleation of small pancreatic tumors[J]. Langenbecks Arch Surg, 2014, 399(3):315–321. doi:10.1007/s00423–014–1171–0.
[4]Brient C, Regenet N, Sulpice L, et al. Risk factors for postoperative pancreatic fistulization subsequent to enucleation[J]. J Gastrointest Surg, 2012, 16(10):1883–1887.
[5]中华医学会外科学分会胰腺外科学组. 胰腺囊性疾病诊治指南(2015版)[J]. 临床肝胆病杂志, 2015, 31(9):1375–1378.doi:10.3969/j.issn.1001–5256.2015.09.003.Group of Pancreas Surgery, Chinese Society of Surgery. Guidelines for the management of pancreatic cystic lesions(2015)[J]. Journal of Clinical Hepatology, 2015, 31(9):1375–1378. doi:10.3969/j.issn.1001–5256.2015.09.003.
[6]Edwin B, Sahakyan MA, Abu Hilal M, et al. Laparoscopic surgery for pancreatic neoplasms: the European association for endoscopic surgery clinical consensus conference[J]. Surg Endosc, 2017,31(5):2023–2041. doi: 10.1007/s00464–017–5414–3.
[7]Goh BK. International guidelines for the management of pancreatic intraductal papillary mucinous neoplasms[J]. World J Gastroenterol,2015, 21(34):9833–9837. doi: 10.3748/wjg.v21.i34.9833.
[8]Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillarymucinous neoplasms and mucinous cystic neoplasms of the pancreas[J].Pancreatology, 2006, 6(1/2):17–32.
[9]Clark OH, Benson AB 3rd, Berlin JD, et al. NCCN Clinical Practice Guidelines in Oncology: neuroendocrine tumors[J]. J Natl Compr Canc Netw, 2009, 7(7):712–747.
[10]刘荣, 赵国栋, 尹注增, 等. 机器人胰腺肿瘤剜除联合主胰管架桥修复术个案报道[J]. 中华腔镜外科杂志:电子版, 2016, 9(6):373–374. doi:10.3877/cma.j.issn.1674–6899.2016.06.014.Liu R, Zhao D, Yin ZZ, et al. Case report of robotic enucleation of pancreatic tumor combined with bridging repair of the main pancreatic duct[J]. Chinese Journal of Laparoscopic Surgery:Electronic Edition, 2016, 9(6):373–374. doi:10.3877/cma.j.issn.1674–6899.2016.06.014.
[11]刘荣, 王子政, 高元兴, 等. 机器人"荣氏"胰腺中段切除术一例报道[J]. 中华腔镜外科杂志:电子版, 2017, 10(5):319–320.doi:10.3877/cma.j.issn.1674–6899.2017.05.023.Liu R, Wang ZZ, Gao YX, et al. Rong's robotic middle segment pancreatectomy: a report of one case[J]. Chinese Journal of Laparoscopic Surgery: Electronic Edition, 2017, 10(5):319–320.doi:10.3877/cma.j.issn.1674–6899.2017.05.023.
[12]Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperativepancreatic fistula: 11 Years After[J]. Surgery, 2017,161(3):584–591. doi: 10.1016/j.surg.2016.11.014.
[13]Fernandez Ranvier GG, Shouhed D, Inabnet WB 3rd. Minimally Invasive Techniques for Resection of Pancreatic Neuroendocrine Tumors[J]. Surg Oncol Clin N Am, 2016, 25(1):195–215. doi:10.1016/j.soc.2015.08.009.
[14]Røsok BI, de Rooij T, van Hilst J, et al. Minimally invasive distal pancreatectomy[J]. HPB (Oxford), 2017, 19(3):205–214. doi:10.1016/j.hpb.2017.01.009.
[15]Gavriilidis P, Lim C, Menahem B, et al. Robotic versus laparoscopic distal pancreatectomy -The first meta-analysis[J]. HPB (Oxford),2016, 18(7):567–574. doi: 10.1016/j.hpb.2016.04.008.
[16]Magge D, Zureikat A, Hogg M, et al. Minimally Invasive Approaches to Pancreatic Surgery[J]. Surg Oncol Clin N Am, 2016,25(2):273–286. doi: 10.1016/j.soc.2015.11.001.
[17]Lianos GD, Christodoulou DK, Katsanos KH, et al. Minimally Invasive Surgical Approaches for Pancreatic Adenocarcinoma:Recent Trends[J]. J Gastrointest Cancer, 2017, 48(2):129–134. doi:10.1007/s12029–017–9934–9.
[18]Welsch T, Distler M, Weitz J. Minimally invasive and robot-assisted surgery for pancreatic cystic tumors[J]. Chirurg, 2017, 88(11):934–943. doi: 10.1007/s00104–017–0496-y.
[19]Tamburrino D, Partelli S, Renzi C, et al. Systematic review and meta-analysis on laparoscopic pancreatic resections for neuroendocrine neoplasms (PNENs)[J]. Expert Rev Gastroenterol Hepatol, 2017, 11(1):65–73.
[20]Liu R, Liu Q, Zhao ZM, et al. Robotic versus laparoscopic distal pancreatectomy: A propensity score-matched study[J]. J Surg Oncol, 2017, 116(4):461–469. doi: 10.1002/jso.24676.
[21]Liu R, Zhang T, Zhao ZM, et al. The surgical outcomes of robotassisted laparoscopic pancreaticoduodenectomy versus laparoscopic pancreaticoduodenectomy for periampullary neoplasms: a comparative study of a single center[J]. Surg Endosc, 2017,31(6):2380–2386. doi: 10.1007/s00464–016–5238–6.