胰腺癌是我国癌症相关死亡的第5位主要原因[1]。2015年,我国胰腺癌新发病例90.1万例,死亡病例79.4万例[1]。胰腺癌也是恶性肿瘤中预后最差的肿瘤类型,患者平均存活时间为6个月,5年生存率仅6%[2]。即便患者在早期可成功诊断,但术后复发率仍很高, 复发与转移仍是影响胰腺癌预后的主要原因[3]。因此,对胰腺癌发生、发展及发病机制作深入探索将为胰腺癌的诊治提供新的思路。长链非编码RNA(Long non-coding RNA,LncRNA)是一类长度超过200 bp的非编码RNA,近几年已成为肿瘤分子生物学研究热点[4]。LncRNA参与多种生物学过程,如细胞分化、衰老、凋亡和转移、化疗耐药性等[5-6]。研究[7-9]发现,LncRNA能通过表观遗传修饰、剪接、RNA降解、翻译后修饰等方式沉默抑癌基因或激活促癌基因,参与肿瘤发生和转移等过程。先前报道,LncRNA BCAR4参与多种肿瘤发生发展过程,如乳腺癌[10]、骨肉瘤[11]、肺癌[12]等。但至今尚无LncRNA BCAR4在胰腺癌中的相关报道。本研究旨研究LncRNA BCAR4对胰腺癌细胞增殖和凋亡的影响,并探讨其机制。
1 材料与方法
1.1 材料
人正常胰腺导管上皮细胞系HPDE6-C7及胰腺癌细胞系AsPC-1、HPAC、BxPC-3、Panc-1均购自美国ATCC细胞库,实验所需的磷酸化哺乳动物雷帕霉素靶蛋白(phosphorylated mammalian target of rapamycin,p-mTOR)、磷酸化核糖体蛋白S6激酶(phosphorylated ribosomal protein S6 kinase,p-P70S6K)、核糖体蛋白S6激酶(ribosomal protein S6 kinase,P70S6K)及哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)一抗均购自美国Santa Cruze公司,二抗购自美国BD公司,RPMI 1640培养基购自上海经科化学科技有限公司,LncRNA BCAR4 siRNA套装购自广州锐博生物科技有限公司,LipofectamineTM 2000转染试剂购自美国BD公司。RT-PCR仪购自美国BD公司,HBS-1096B酶标仪购自南京德铁实验设备有限公司,蛋白免疫印迹电泳设备购自美国Bio-Rad公司。
1.2 实验方法
1.2.1 细胞培养 人正常胰腺导管上皮细胞系HPDE6-C7及胰腺癌细胞系AsPC-1、HPAC、BxPC-3、Panc-1均种植于RPMI 1640培养基, 并培养于37 ℃、5% CO2培养箱中,于48 h后消化传代,实验所用的细胞均为对数生长期细胞。
1.2.2 细胞转染及分组 取AsPC-1细胞系分成3组,si-BCAR4组、阴性对照组及空白对照组,并制备终浓度为80 nmol/L的LncRNA BCAR4siRNA脂质体复合物,将AsPC-1细胞系以每孔2×105个接种于6孔板上,待细胞生长至70%~80%时,更换无血清培养基,si-BCAR4组和阴性对照组经LipofectamineTM 2000分别转染BCAR4 siRNA序列和阴性对照siRNA序列,以无转染的AsPC-1细胞为空白对照。BCAR4 siRNA序列正义链:5'-GGG ACU UGA GUU AUG UUG GUG GCU A-3';反义链:5'-UAG CCA CCA ACA UAA CUC AAG UCC C-3'。阴性对照siRNA序列正义链:5'-UUC UCC GAA CGU GUC ACG U-3';反义链:5'-ACG UGA CAC GUU CGG AGA A-3'。
1.2.3 qRT-PCR检测 RNA的提取:收集si-BCAR4组、阴性对照组及空白对照组3组细胞,每孔不少于1×106个细胞,用All-in-One miRNA抽提试剂盒提取总RNA,取5 μg总RNA行反转录合成cDNA,以cDNA为模板,GAPDH为内参,LncRNA BCAR4引物序列正向:5'-ACA GCA GCT TGT TGC TCA TCT-3', 反 向:5'-TTG CCT TGG GGA CAG TTC AC-3';GAPDH引物序列正向:5'-GCT CTC TGC TCC TCC TGT TC-3'; 反向:5'-ACG ACC AAA TCC GTT GAC TC-3'。 行qRT-PCR反应。反应条件:95 ℃预变性30 s,95℃5s,60 ℃ 20 s,共40个循环,使用Bio-Rad real-time PCR仪自带软件分析样本的循环阈值(cycle threshold,CT),采用 2−ΔΔCt方法定量,计算LncRNA BCAR4的相对表达量。
1.2.4 细胞增殖能力测定 CCK-8法测定si-BCAR4组、阴性对照组及空白对照组3组细胞增殖能力,将si-BCAR4组、阴性对照组及空白对照组3组细胞消化成单细胞悬液后,以2×103个/孔将3组细胞种植于96孔板上,每个孔按200 µL的体积上样,经0、1、2、3、4 d培养后,20 µL CCK-8溶液加入于每孔中去,继续培养1 h后,在450 nm波长下,用酶标仪测定各孔吸光值,以时间为横坐标,吸光值为纵坐标绘制细胞增殖曲线。
1.2.5 细胞凋亡测定 采用流式细胞术测定si-BCAR4组和阴性对照组两组细胞凋亡率,染色采用Annexin V/PI,将si-BCAR4组和阴性对照组两组细胞消化后,结合缓冲液重悬混匀后,加入Annexin V抗体,避光染色10 min后加入适量PBS溶液及PI染料,流式细胞仪检测Annexin V阳性细胞比例来确定细胞凋亡率。
1.2.6 Western blot检测 采用Western blot法,将si-BCAR4组和阴性对照组两组细胞裂解、变性后,上样量为每孔30 μg蛋白,浓缩胶条件为50 min 80 V,分离胶条件为100 min 100 V,常规 转 膜,加入p-mTOR、mTOR、p-P70S6K及P70S6K一抗,一抗浓度为1:200,于4 ℃孵育过夜,二抗(1:1 000)经37 ℃孵育4 h后,PBST漂洗3次,在ECL发光液下显影,Quantity One 1-D分析目标蛋白灰度值,目标蛋白相对表达量=目标蛋白灰度值/GAPDH灰度值,实验重复3次,取平均值。
1.3 统计学处理
采用SPSS 20.0统计软件行数据分析,计量资料以均数±标准差(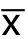 ±s)表示,两组间的比较采用t检验,3组比较先用方差分析,有意义时,两两比较再用LSD-t检验,P<0.05为差异有统计学意义。
±s)表示,两组间的比较采用t检验,3组比较先用方差分析,有意义时,两两比较再用LSD-t检验,P<0.05为差异有统计学意义。
2 结 果
2.1 LncRNA BCAR4在胰腺癌细胞系中的表达
qRT-PCR结果显示,人正常胰腺导管上皮细胞系HPDE6-C7的LncRNA BCAR4的相对表达量为1.0±0.03,胰腺癌细胞系AsPC-1、HPAC、BxPC-3、Panc-1中LncRNA BCAR4的相对表达量分别为5.38±0.27(P<0.001)、4.05±0.22(P<0.01)、3.49±0.15(P<0.05)及2.94±0.11(P<0.05),LncRNA BCAR4在胰腺癌细胞系中呈高表达(图1)。
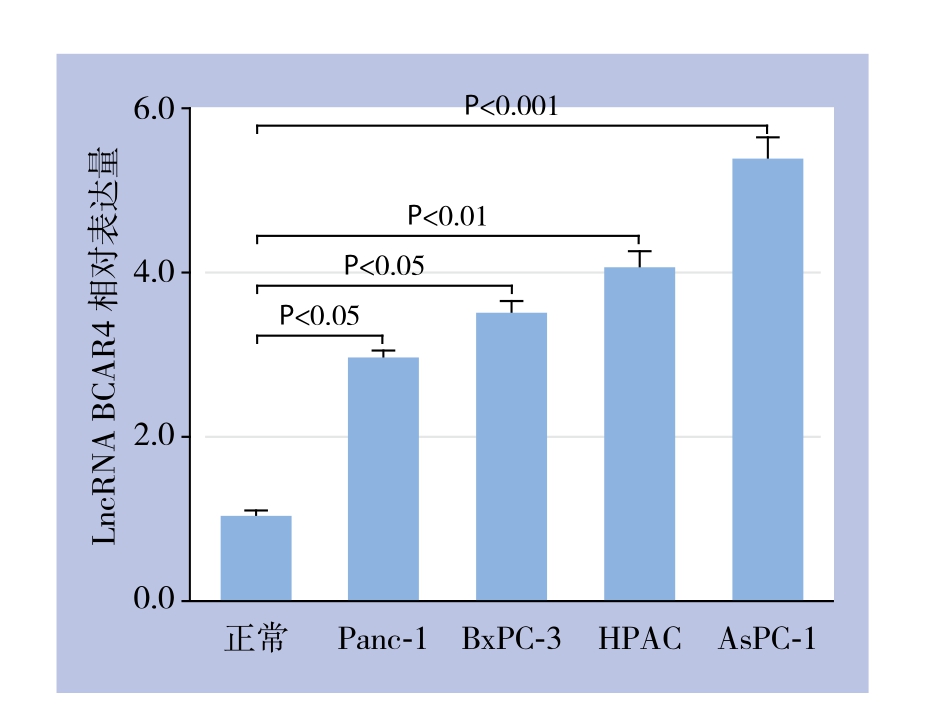
图1 LncRNA BCAR4在不同胰腺癌细胞系及正常胰腺导管上皮细胞系中的表达
Figure 1 The expression of LncRNA BCAR4 in different pancreatic carcinoma cell lines and normal pancreatic duct epithelial cells
2.2 抑制LncRNA BCAR4表达对AsPC-1细胞增殖的影响
qRT-PCR结果显示,转染48 h后,si-BCAR4组LncRNA BCAR4相对表达量为0.26±0.03,阴性对照组为1.03±0.05,空白对照组为1.0±0.03,si-BCAR4组LncRNA BCAR4相对表达量低于阴性对照组和空白对照组(均P<0.001)(图2A);CCK-8实验结果显示,转染0、24、48、72、96 h后,si-BCAR4组与阴性对照组的OD450nm值分别为[(0.33±0.04) vs.( 0.32±0.04),P>0.05]、[(0.49±0.05) vs.( 0.52±0.06),P>0.05],[(0.72±0.07)vs.( 0.84±0.08),P>0.05],[(0.97±0.09)vs (1.28±0.10),P<0.05]、[(1.31±0.10)vs.( 1.97±0.14),P<0.01],空白对照组与阴性对照组各时间点OD450nm值差异均无统计学意义(均P>0.05)(图2B)。

图2 BCAR4 siRNA转染对AsPC-1细胞LncRNA BCAR4表达及增殖的影响 A:LncRNA BCAR4表达检测;B:细胞增殖检测
Figure 2 Effect of BCAR4 siRNA transfection on LncRNA BCAR4 expression and proliferation in AsPC-1 cells A: Determination of LncRNA BCAR4 expression; B: Determination of cell proliferation
2.3 抑制LncRNA BCAR4表达对AsPC-1细胞凋亡的影响
流式细胞检测结果显示,转染4 8 h后,si-BCAR4组细胞凋亡率为(15.63±3.19)%,阴性对照组为(4.84±1.67)%,si-BCAR4组细胞凋亡率高于阴性对照组(P<0.01)(图3)。
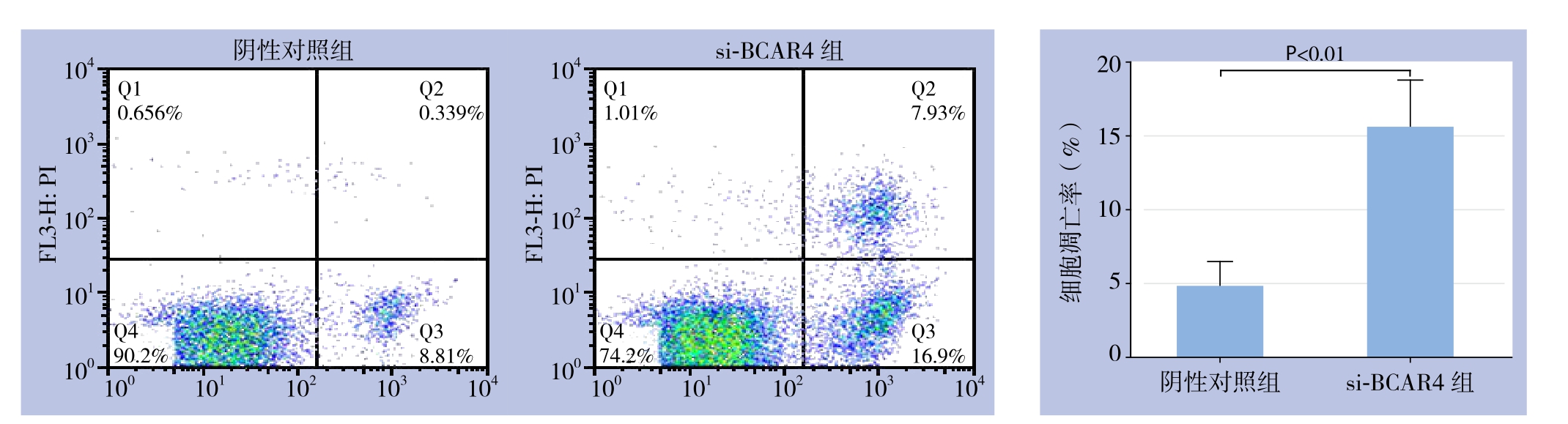
图3 细胞凋亡检测
Figure 3 Apoptosis analysis
2.4 抑制LncRNA BCAR4表达对AsPC-1细胞mTOR信号通路的影响
Western blot结果显示,si-BCAR4组p-mTOR蛋白相对表达量为0.37±0.04,阴性对照组为1.0±0.05,si-BCAR4组p-mTOR蛋白相对表达量低于阴性对照组(P<0.01);si-BCAR4组p-P70S6K蛋白相对表达量为0.43±0.04,阴性对照组为1.0±0.08,si-BCAR4组p-P70S6K蛋白相对表达量低于阴性对照组(P<0.01);两组mTOR与P70S6K相对表达量均无统计学差异(均P>0.05)(图4)。
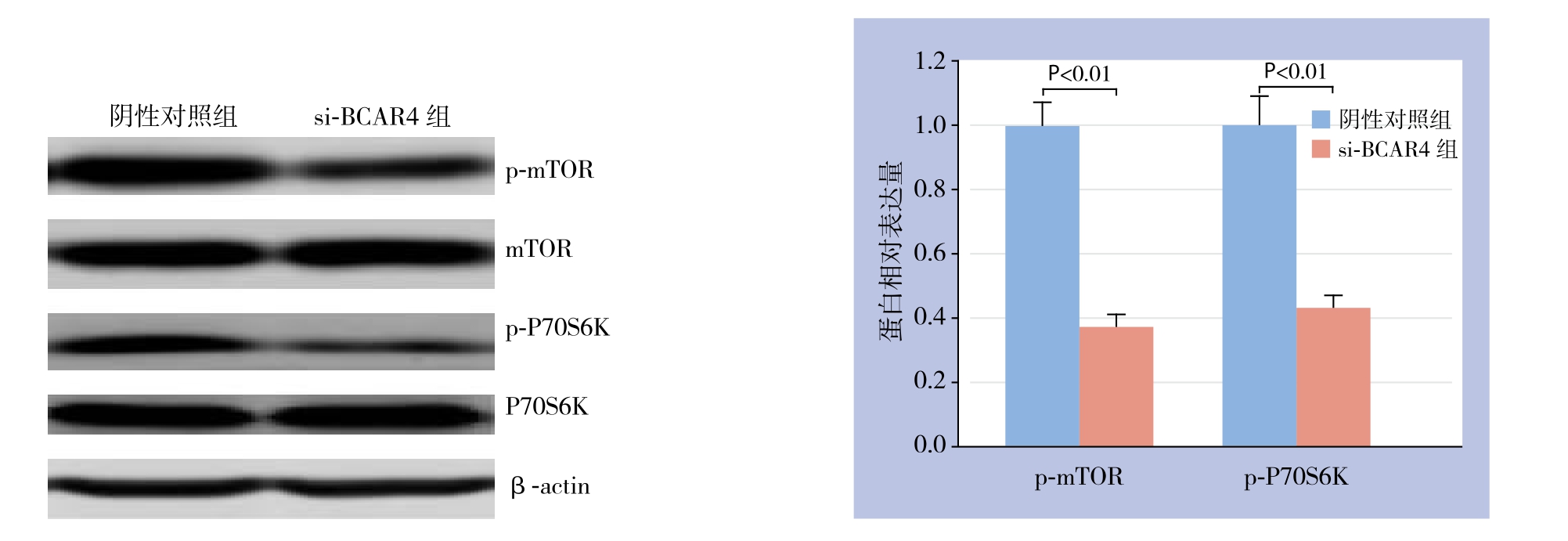
图4 Western blot检测mTOR信号通路相关蛋白
Figure 4 Western blot analysis for mTOR signaling pathway related proteins
3 讨 论
近年来,胰腺癌发病率呈逐渐上升趋势,已成为我国常见的恶性肿瘤之一[1]。胰腺导管腺癌是胰腺癌的主要病理类型,它由腺泡导管化生或肿瘤前体病变发展而来[13-14]。在过去30年里,其他胃肠道恶性肿瘤患者的生存率呈持续上升趋势,但胰腺癌患者的预后并无显著改善,超过80%的患者在手术切除后会复发[15-17]。
当前研究[18-20]表明,胰腺癌的发生发展受基因变异及表观遗传学的影响。越来越多的证据表明,LncRNA常在肿瘤发展、转移阶段呈异常表达。Godinho等[10]指出LncRNA BCAR4在27%的原发性乳腺癌患者肿瘤中表达,LncRNA BCAR4高表达与患者无进展生存率相关。Chen等[11]发现LncRNA BCAR4高表达于骨肉瘤组织,过表达的LncRNA BCAR4与肿瘤大小、分期、肺转移及预后不良相关,LncRNA BCAR4敲除后可通过调控锌指蛋白2的表达抑制骨肉瘤增殖和迁移。Bae等[21]检测了LncRNA BCAR4在原发性肺癌组织和癌旁组织中的表达差异,发现LncRNA BCAR4在71%的肺癌细胞中高表达,过表达LncRNA BCAR4后肺癌细胞生长能力增强,而采用siRNA技术敲除肿瘤细胞LncRNA BCAR4表达后,细胞克隆形成能力明显受抑制。本研究通过荧光定量PCR检测了LncRNA BCAR4在胰腺癌细胞系和正常胰腺细胞中的表达差异,证实LncRNA BCAR4在胰腺癌细胞中高表达。随后,采用siRNA技术行功能研究,CCK-8检测表明LncRNA BCAR4敲除后胰腺癌细胞增殖能力明显受抑制,流式细胞术检测发现该基因沉默后肿瘤细胞凋亡率升高。这些结果表明,LncRNA BCAR4是作为促癌基因参与胰腺癌致瘤过程,可影响肿瘤细胞增殖和凋亡过程。
有报道[22]指出,LncRNA BCAR4通过激活mTOR信号途径促进软骨细胞增殖和迁移。因此,我们通过Western blot技术发现胰腺癌细胞敲除LncRNA BCAR4后p-mTOR和p-P70S6K蛋白表达降低,mTOR和P70S6K蛋白表达上升。mTOR是一种典型的丝氨酸/苏氨酸激酶,在调节细胞增殖、生长、分化、迁移和生存中起核心作用[23]。它通过磷酸化激活下游效应物4EBP1和P70S6K激酶参与细胞生长、周期进展(如诱导G1期基因细胞周期蛋白D的表达)和细胞代谢等。mTOR-p70S6K信号通路常在多数人类肿瘤细胞系中被激活,如胰腺癌和小细胞肺癌等[24]。研究[25]发现,细胞质磷酸化mTOR(p-mTOR)的表达与胃癌肿瘤侵袭深度呈正相关,高表达细胞质p-mTOR的患者无复发生存率和总生存率较低。在本研究,本研究发现敲除LncRNA BCAR4的胰腺癌细胞中活性mTOR(p-mTOR)及活性p70S6K(p-P70S6K)蛋白表达水平降低,表明mTOR-p70S6K信号途径因LncRNA BCAR4敲除而受抑制。推测,LncRNA BCAR4沉默抑制胰腺癌细胞增殖和促进凋亡,与mTOR-p70S6K信号通路失调有一定关联。
综上,LncRNA BCAR4在胰腺癌细胞系中高表达,沉默其表达可抑制胰腺癌细胞增殖、促进凋亡,其机制可能与mTOR-p70S6K信号通路失调有关,这为胰腺癌发生发展的分子机制研究提供了新的思路,也为肿瘤诊断、治疗、预后提供新的策略。
参考文献
[1]Chen W, Zheng R, Baade PD, et al. Cancer statistics in China,2015[J]. CA Cancer J Clin, 2016, 66(2):115–132. doi: 10.3322/caac.21338.
[2]Ouyang H, Gore J, Deitz S, et al. microRNA-10b enhances pancreatic cancer cell invasion by suppressing TIP30 expression and promoting EGF and TGF-β actions[J]. Oncogene, 2014,33(38):4664–4674. doi: 10.1038/onc.2013.405.
[3]Jones OP, Melling JD, Ghaneh P. Adjuvant therapy in pancreatic cancer[J]. World J Gastroenterol, 2014, 20(40):14733–14746. doi:10.3748/wjg.v20.i40.14733.
[4]Sun M, Nie F, Wang Y, et al. LncRNA HOXA11-AS promotes proliferation and invasion of gastric cancer by scaffolding the chromatin modification factors PRC2, LSD1, and DNMT1[J].Cancer Res, 2016, 76(21):6299–6310.
[5]Yarmishyn AA, Kurochkin IV. Long noncoding RNAs: a potential novel class of cancer biomarkers[J]. Front Genet, 2015, 6:145. doi:10.3389/fgene.2015.00145.
[6]Shi SJ, Wang LJ, Yu B, et al. LncRNA-ATB promotes trastuzumab resistance and invasion-metastasis cascade in breast cancer[J].Oncotarget, 2015, 6(13):11652–11663.
[7]Kim HS, Minna JD, White MA. GWAS meets TCGA to illuminate mechanisms of cancer predisposition[J]. Cell, 2013, 152(3):387–389. doi: 10.1016/j.cell.2013.01.027.
[8]Rinn JL. lncRNAs: linking RNA to chromatin[J]. Cold Spring Harb Perspect Biol, 2014, 6(8). pii: a018614. doi: 10.1101/cshperspect.a018614.
[9]Iguchi T, Uchi R, Nambara S, et al. A long noncoding RNA,lncRNA-ATB, is involved in the progression and prognosis of colorectal cancer[J]. Anticancer Res, 2015, 35(3):1385–1388.
[10]Godinho M, Meijer D, Setyono-Han B, et al. Characterization of BCAR4, a novel oncogene causing endocrine resistance in human breast cancer cells[J]. J Cell Physiol, 2011, 226(7):1741–1749. doi:10.1002/jcp.22503.
[11]Chen F, Mo J, Zhang L. Long noncoding RNA BCAR4 promotes osteosarcoma progression through activating GLI2-dependent gene transcription[J]. Tumour Biol, 2016, 37(10):13403–13412.
[12]van Agthoven T, Dorssers LC, Lehmann U, et al. Breast cancer anti-estrogen resistance 4 (BCAR4) drives proliferation of IPH-926 lobular carcinoma cells[J]. PLoS One, 2015, 10(8):e0136845. doi:10.1371/journal.pone.0136845.
[13]Aichler M, Seiler C, Tost M, et al. Origin of pancreatic ductal adenocarcinoma from atypicalflat lesions: a comparative study in transgenic mice and human tissues[J]. J Pathol, 2012, 226(5):723–734. doi: 10.1002/path.3017.
[14]Morris JP 4th, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma[J]. Nat Rev Cancer, 2010, 10(10):683–695. doi:10.1038/nrc2899.
[15]Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-theart treatments to promising novel therapies[J]. Nat Rev Clin Oncol,2015, 12(6):319–334. doi: 10.1038/nrclinonc.2015.53.
[16]刘江, 吉顺荣, 徐近, 等. 临界可切除胰腺癌的诊疗策略[J]. 中国普通外科杂志, 2017, 26(9):1089–1092. doi:10.3978/j.issn.1005–6947.2017.09.002.Liu J, Ji SR, Xu J, et al. Diagnosis and treatment strategies for borderline resectable pancreatic cancer [J]. Chinese Journal of General Surgery, 2017, 26(9):1089–1092. doi:10.3978/j.issn.1005–6947.2017.09.002.
[17]黄耿文, 宁彩虹, 申鼎成, 等. 《日本胰腺协会胰腺癌临床实践指南(2016)》解读[J]. 中国普通外科杂志, 2017, 26(9):1093–1096. doi:10.3978/j.issn.1005–6947.2017.09.003.Huang GW, Ning CH, Shen DC, et al. Interpretation of Clinical Practice Guidelines for Pancreatic Cancer 2016 from the Japan Pancreas Society[J]. Chinese Journal of General Surgery, 2017,26(9):1093–1096. doi:10.3978/j.issn.1005–6947.2017.09.003.
[18]Li Z, Dong M, Fan D, et al. LncRNA ANCR down-regulation promotes TGF-β-induced EMT and metastasis in breast cancer[J]. Oncotarget, 2017, 8(40):67329–67343. doi: 10.18632/oncotarget.18622.
[19]魏伟, 杨波, 唐翎. 长链非编码RNA XIST在胰腺癌中的表达及意义[J]. 中国普通外科杂志, 2017, 26(3):304–310. doi: 10.3978/j.issn.1005–6947.2017.03.006.Wei W, Yang B, Tang L. Expression of long noncoding RNA XIST in pancreatic cancer and its significance[J]. Chinese Journal of General Surgery, 2017, 26(3):304–310. doi: 10.3978/j.issn.1005–6947.2017.03.006.
[20]陈辉星, 陈实, 李小燕, 等. 胰腺癌中Ring1B、LSD1及P16表达及其与预后的关系[J]. 中国普通外科杂志, 2017, 26(9):1148–1154.doi:10.3978/j.issn.1005–6947.2017.09.011.Chen HX, Chen S, Li XY, et al. Expressions of Ring1B, LSD1 and P16 in pancreatic cancer and their prognostic impacts[J]. Chinese Journal of General Surgery, 2017, 26(9):1148–1154. doi:10.3978/j.issn.1005–6947.2017.09.011.
[21]Bae K, Lee M, Yoon D, et al. Breast cancer anti-estrogen resistance 4 (BCAR4) is a novel oncogene in lung cancer [J]. Cancer Res,2017, Abstract 3137. doi: 10.1158/1538–7445.
[22]Shui X, Zhou C, Lin W, et al. Long non-coding RNA BCAR4 promotes chondrosarcoma cell proliferation and migration through activation of mTOR signaling pathway[J]. Exp Biol Med (Maywood), 2017, 242(10):1044–1050. doi:10.1177/1535370217700735.
[23]Huang S, Houghton PJ. Targeting mTOR signaling for cancer therapy[J]. Curr Opin Pharmacol, 2003, 3(4):371–377.
[24]Gao N, Flynn DC, Zhang Z, et al. G1 cell cycle progression and the expression of G1 cyclins are regulated by PI3K/AKT/mTOR/p70S6K1 signaling in human ovarian cancer cells[J]. Am J Physiol Cell Physiol, 2004, 287(2):C281–291.
[25]Murayama T, Inokuchi M, Takagi Y, et al. Relation between outcomes and localisation of p-mTOR expression in gastric cancer[J]. Br J Cancer, 2009, 100(5):782–788. doi: 10.1038/sj.bjc.6604915.