胆囊癌是胆道系统最常见的恶性肿瘤[1],其恶性程度高、临床症状出现迟、早期诊断困难。不仅如此,胆囊癌的进展极快,胆囊癌患者术后的5年生存率仅为5%~12%[2]。乙酰肝素酶(heparanase)作为哺乳动物体内唯一可以特异性切割细胞表面HS链的酶,在人类的肿瘤[3]、炎症[4]、血管增生[5]、先兆子痫[6]、朊病毒病[7]等多种病理生理过程当中发挥着重要的作用。一些研究发现,乙酰肝素酶可以促进肿瘤的发生和发展,因此,乙酰肝素酶的抑制剂作为潜在的抗肿瘤药物,受到极大的关注[8]。作为细胞表面的黏蛋白多糖,多配体蛋白聚糖1(syndecan-1)同样是一种与肿瘤的发生密切相关的生物大分子蛋白。多配体蛋白聚糖1参与调控多项生命活动,包括聚集淋巴细胞、调节免疫机制和重塑细胞基质等,尤为重要的是可以对肿瘤细胞的侵袭和黏附起到调节作用[9]。已经有研究证实,在消化道肿瘤当中,乙酰肝素酶可以调低多配体蛋白聚糖1的水平,而多配体蛋白聚糖1水平的降低则引起肿瘤的生长加速[10-14]。但在胆囊癌细胞中是否存在相同的情况,还没有相关试验进行证实。本研究就两者在胆囊癌细胞中的关系进行探讨。
1 材料与方法
1.1 实验材料
人胆囊癌细胞系GBC-SD购自上海中国科学院生物科学研究院生物细胞库;DMEM培养基购自美国Hyclone公司;胎牛血清购于四季青公司;1:250胰蛋白酶,RPMI-1640培养基,FBS,TRIzol溶液,LipofectamineTM2000脂质体购于美国Invitrogen公司;PCR引物,分子标志物和dNTPs购于上海生工生物有限公司;逆转录元件,限制性内切酶BglII和SalI,T4 DNA连接酶,BCA蛋白定量和实时qPCR元件购于MBI Fermentas中国有限公司;质粒pcDNA3.1购于本诺生物技术有限公司,PCR提纯和质粒提取元件购自美国Axygen生物公司;乙酰肝素酶单克隆一抗,辣根过氧化物酶兔抗IgG购自博士德生物科技有限公司;基质膜购自美国BD生物科学公司。
1.2 质粒构建和验证
构建乙酰肝素酶过表达的载体。乙酰肝素酶基因序列由GeneBank检索得出,依据乙酰肝素酶序列(基因ID:ENSG00000225609.1)合成单链脱氧核苷酸,再通过变性和退火转变成双链DNA。将特殊序列嵌入pGPU6/GFP/Neo载体。将载体转染入大肠埃希菌,于37 ℃ 室温下在含有庆大霉素的培养基上对大肠埃希菌进行隔夜培养。将大肠埃希菌质粒中的质粒进行分离并确定其DNA序列,将具有正确序列的质粒用于下一步实验。
1.3 质粒转染
根据操作说明在脂质体介导下使用重组质粒转染人胆囊癌细胞GBC-SD。在转染24 h后使用反式荧光显微镜测定其转染率。在加入10%FBS的DMEM培养基中对被转染的细胞系进行保存和培养。
1.4 RT-PCR检测
使用Primer Express软件从GeneBank数据库中检索出乙酰肝素酶、多配体蛋白聚糖1、β-actin引物的基因序列,以此构建引物。乙酰肝素酶引物正向:5'-AGC CTC GAA GAA AGA CGG-3';反向:5'-GTA GTG ATG CCA TGT AAC TGA ATC-3'。多配体蛋白聚糖1引物正向:5'-GGA AAG AGG TGC TGG GAG GG-3';反向:5'-TTG GTG GGC TTC TGG TAG GC-3'。β-actin引物正向:5'-GGA AAT CGT GCG TGA CAT TAA G-3';反向:5'-GGA AAT CGT GCG TGA CAT TAA G-3'。通过TRIzol提取液将细胞中全部乙酰肝素酶mRNA提取出,然后依据操作手册实施RT-PCR。
1.5 Western blot检测
使用二烷基硫酸钠聚丙烯酰胺凝胶电泳(SDS-PAGE)从细胞中分离出等量的乙酰肝素酶或多配体蛋白聚糖1。将乙酰肝素酶抗体或多配体蛋白聚糖1 抗体和β-actin抗体按1:500比例进行稀释。以辣根过氧化物酶(HRP)标记二抗,使用增强化学发光(ECL)Western blot检测系统检测条带。
1.6 细胞侵袭和转移试验
Transwell侵袭和转移试验的具体实施方法在之前的文献[15]中已经具体讲述过。将小室(8 mm孔隙小格)上层的24个小格中均匀的加入无血清的RPMI-1640培养基,分别取5×104的GBC-SD细胞和GBD-SD细胞放置在小室上层中,每个小格都加入了琼脂凝胶。对于转移试验,上层小室中不加入基质胶。含有10%FBS的RPMI-1640加入下层小室作为化学诱导物。经过24 h孵育后,小室上层表面的细胞被清理掉;而侵袭穿透入基质胶膜的细胞则被多聚甲醛固定下来并被结晶紫染色。通过在转化显微镜200倍视野下每层膜随机选择5个视野从而将侵袭细胞的数目计算出来。
1.7 统计学处理
数据以均数±标准差( ±s)表示,用t检验及单因素方差分析比较组间差异,P<0.05作为差异有统计学意义。
±s)表示,用t检验及单因素方差分析比较组间差异,P<0.05作为差异有统计学意义。
2 结 果
2.1 重组载体与转染率的鉴定
序列检测结果显示DNA载体序列和所设计的序列相同。未监测到删除、嵌入或突变。在转染后48 h采用免疫荧光显象法观测转染重组质粒后的细胞,重组质粒的平均转染率为81.6%。
2.2 乙酰肝素酶表达量检测
经转染的细胞中乙酰肝素酶mRNA和蛋白含量明显高于未转染细胞中乙酰肝素酶蛋白和mRNA的含量(P<0.05)(图1)。
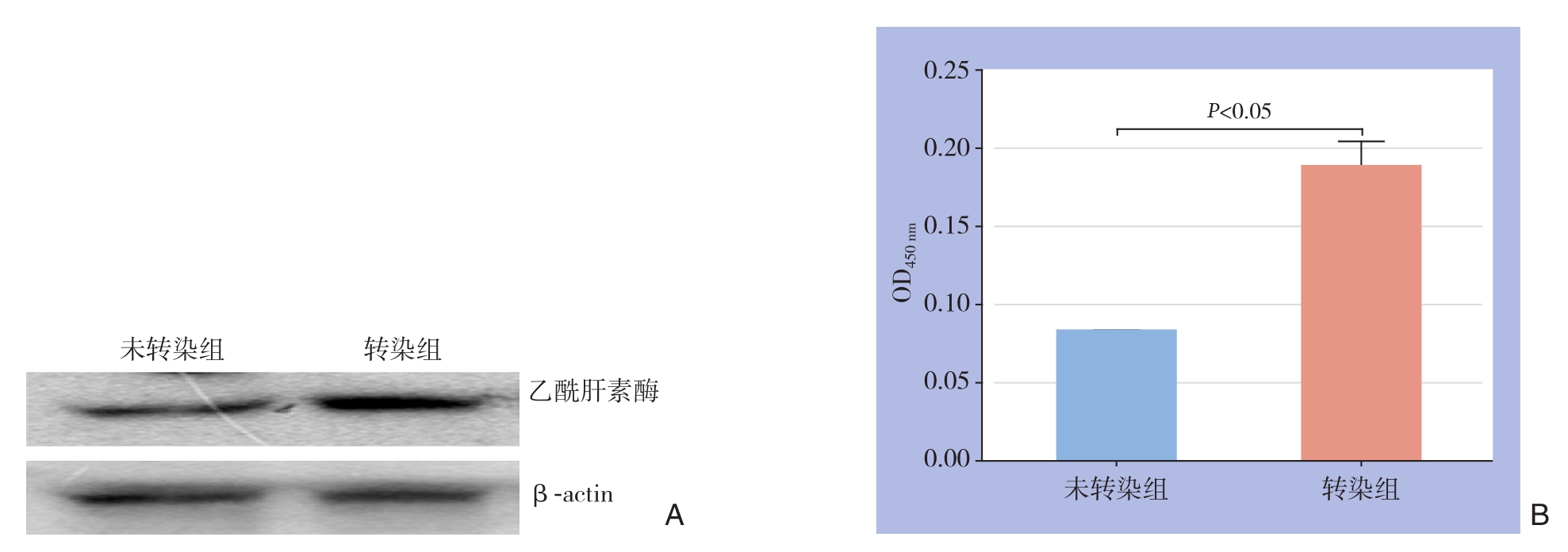
图1 乙酰肝素酶的表达量检测 A:乙酰肝素酶蛋白表达量检测;B:乙酰肝素酶mRNA表达量检测
2.3 多配体蛋白聚糖1表达量检测
经转染的细胞中多配体蛋白聚糖1蛋白与mRNA含量均明显低于未转染细胞,RT-PCR结果定量分析显示差异有统计学意义(P<0.05)(图2)。
2.4 细胞侵袭和转移能力检测
经转染的细胞的侵袭细胞数与转移的细胞数均明显高于未转染细胞(均P<0.05)(图3)。
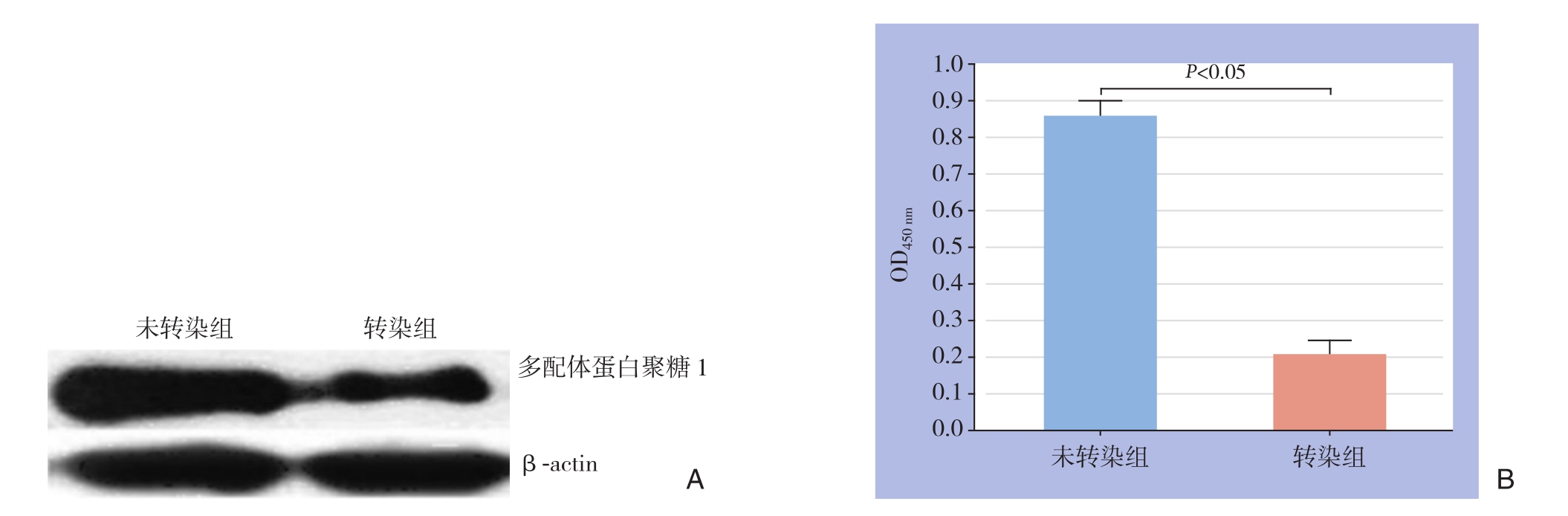
图2 多配体蛋白聚糖1的表达量检测 A:多配体蛋白聚糖1 蛋白表达量检测;B:多配体蛋白聚糖1 mRNA表达量检测
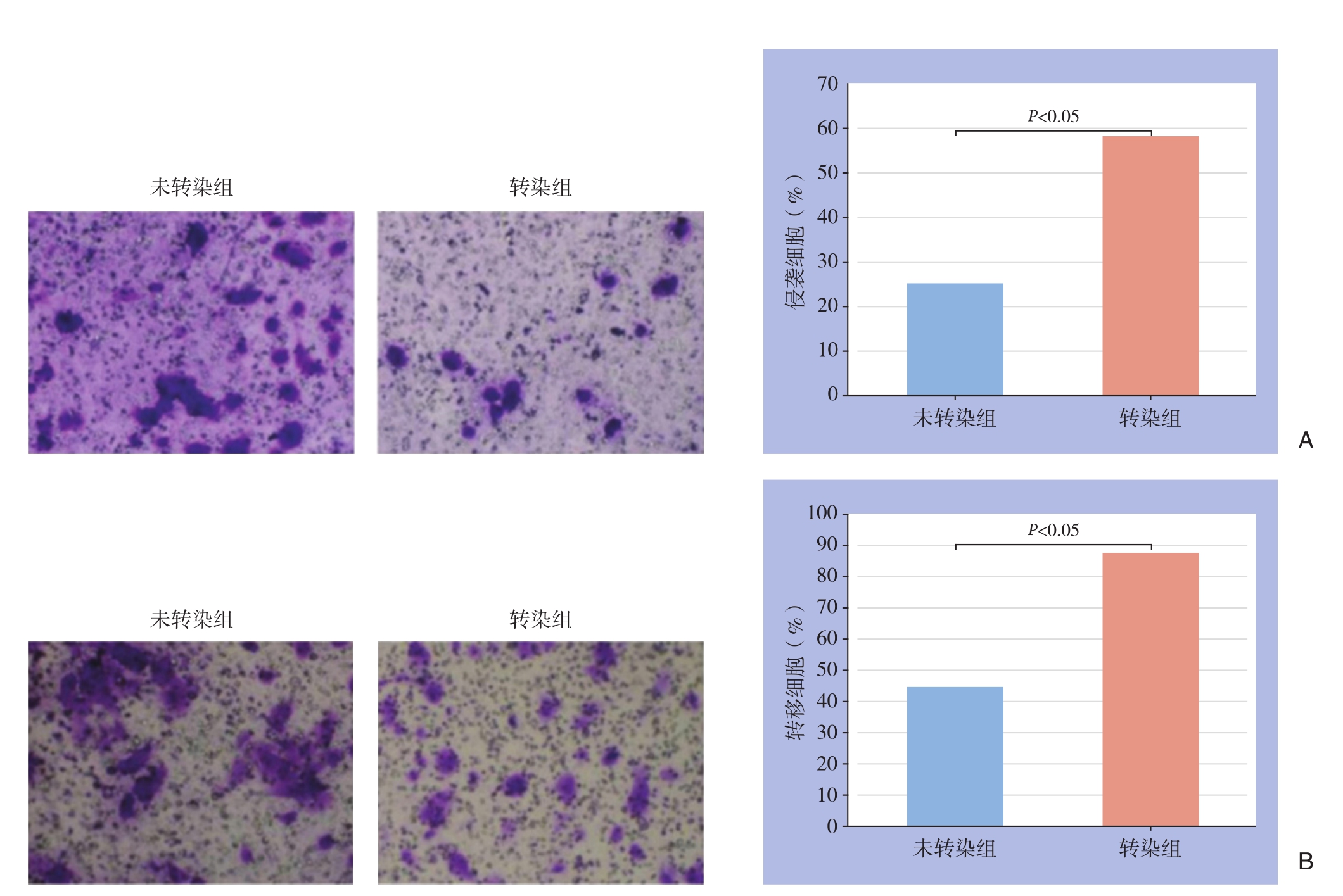
图3 细胞侵袭性和转移能力检测 A:侵袭的细胞数检测;B:转移的细胞数检测
3 讨 论
乙酰肝素酶此前已经被广泛地研究过[16]。有证据[17-27]表明,乙酰肝素酶对肿瘤的侵袭和转移过程中起到关键的作用。为了证明乙酰肝素酶诱导恶性转化的能力,本研究采用重组DNA技术构建出乙酰肝素酶基因过表达的载体,并转染胆囊癌细胞。结果显示,过表达的乙酰肝素酶降低了多配体蛋白聚糖1 的含量,即多配体蛋白聚糖1蛋白的含量与乙酰肝素酶的含量呈负向关系,这与其他相关的研究结果基本一致[9, 28-29]。
本研究结果表明,肿瘤细胞中乙酰肝素酶可以调低多配体蛋白聚糖1表达从而增强胆囊癌细胞的侵袭性和转移能力,提示出一种新的乙酰肝素酶增强肿瘤细胞侵袭性的机制。尽管乙酰肝素酶和乙酰肝素蛋白黏多糖在肿瘤细胞中有着多种作用,最重要的一种作用可能是乙酰肝素酶-多配体蛋白聚糖1在肿瘤和其他疾病中的促血管增生作用。HS和VEGF是肿瘤血管形成中重要的调节剂。研究[15]结果表明,乙酰肝素酶可以增加体内VEGF的含量,提示乙酰肝素酶和多配体蛋白聚糖1是调控肿瘤微环境和促进血管增生的重要物质。
有越来越多的证据表明,乙酰肝素酶可以上调基因的表达,促进肿瘤转移和血管增生、糖代谢、免疫反映、炎症和动脉粥样硬化[30-40]。例如,Nadir等[41]发现在血管上皮细胞和肿瘤细胞中乙酰肝素酶可以诱导组织因子表达。Yang等[42]发现乙酰肝素酶可以通过上调NF-κB配体(RANKL)的受体激动剂在多种骨髓瘤中促进系统性骨质溶解。在2011年,Ramani等[43]发现乙酰肝素酶通过促进肝细胞生长因子(HGF)的表达和活动在促进HGF的信号转导中起到重要作用。在2012年,Cohen-Kaplan等[44]发现乙酰肝素酶可以促进转录反应的信号转导和激活因子(STAT)的磷酸化。不仅如此,Masola等[45]在2014年报道了乙酰肝素酶通过调控转化生长因子β(TGF-β)的表达可以在肾纤维化的过程中起到关键作用。这些研究都表明乙酰肝素酶在细胞核外可以发挥功能,并在调节转录过程中起到关键作用。多配体蛋白聚糖1,一种黏多糖蛋白,在肿瘤的生长和转移发挥着重要的作用,并影响肿瘤的侵袭能力。之前的研究显示,多配体蛋白聚糖1促进骨髓瘤的生长、血管增生和肿瘤的转移[9, 12]。
尽管乙酰肝素酶的调控机制仍是一个有极高价值探讨的问题,本项研究的主要结论是和大多数肿瘤一样,在胆囊癌细胞中,乙酰肝素酶抑制多配体蛋白聚糖1的表达而增强肿瘤细胞的侵袭性,这提供了一个新的视野去了解乙酰肝素酶如何激活肿瘤的侵袭和转移;而乙酰肝素酶抑制剂将有望成为治疗肿瘤的新的药物。
[1]巩鹏,刘鹏,张贤彬,等.意外胆囊癌诊断与治疗的多中心回顾性研究(附223例报告)[J].中华消化外科杂志,2018,17(3):252–256.doi:10.3760/cma.j.issn.1673–9752.2018.03.008.Gong P,Liu P,Zhang XB,et al.Diagnosis and treatment of unexpected gallbladder carcinoma: a multicenter retrospective study(A report of 223 cases)[J].Chinese Journal of Genaral Surgery,2018,17(3):252–256.doi: 10.3760/ cma.j.issn.1673–9752.2018.03.008.
[2]刘立业,仝林虎.胆囊癌治疗的研究进展[J].中国普通外科杂志,2018,27(8):1048–1053.doi:10.3978/j.issn.1005–6947.2018.08.015.Liu LY,Tong LH.Progress in the treatmentof gallbladder carcinoma[J].Chinese Journal of Genaral Surgery,2018,27(8):1048–1053.doi:10.3978/j.issn.1005–6947.2018.08.015.
[3]Leiser Y,Abu-El-Naaj I,Sabo E,et al.Prognostic value of heparanase expression and cellular localization in oral cancer[J].Head Neck,2011,33(6):871–877.doi: 10.1002/hed.21545.
[4]Li JP,Vlodavsky I.Heparin,heparan sulfate and heparanase in inflammatory reactions[J].Thromb Haemost,2009,102(5):823–828.doi: 10.1160/TH09–02–0091.
[5]Nadir Y,Brenner B.Heparanase coagulation and cancer progression[J].Best Pract Res Clin Haematol,2009,22(1):85–92.doi: 10.1016/j.beha.2008.12.004.
[6]Nadir Y,Brenner B.Heparanase procoagulant effects and inhibition by heparins[J].Thromb Res,2010,125(Suppl 2):S72–76.doi:10.1016/S0049–3848(10)70018–9.
[7]Fraser PE.Prions and prion-like proteins[J].J Biol Chem,2014,289(29):19839–19840.doi: 10.1074/jbc.R114.583492.
[8]Baraz L,Haupt Y,Elkin M,et al.Tumor suppressor p53 regulates heparanase gene expression[J].Oncogene,2006,25(28):3939–3947.doi: 10.1038/sj.onc.1209425.
[9]Teng HF,Aquino RS,Pyong WP.Molecular functions of syndecan-1 in disease[J].Matrix Biol,2012,31(1):3–16.doi:10.1016/j.matbio.2011.10.001.
[10]Lee H,Kim Y,Choi Y,et al.Syndecan-2 cytoplasmic domain regulates colon cancer cell migration via interaction with syntenin-1[J].Biochem Biophys Res Commun,201,409(1):148–153.doi:10.1016/j.bbrc.2011.04.135.
[11]Peretti T,Waisberg J,Mader AM,et al.Heparanase-2,syndecan-1,and extracellular matrix remodeling in colorectal carcinoma[J].Eur J Gastroenterol Hepatol,2008,20(8):756–765.doi: 10.1097/MEG.0b013e3282fc2649.
[12]Giordano RJ.Heparanase-2 and syndecan-1 in colon cancer: the ugly ducklings or the beautiful swans?[J]Eur J Gastroenterol Hepatol,2008,20(8):716–718.doi: 10.1097/MEG.0b013e3282fc2660.
[13]Yang Y,Macleod V,Miao HQ,et al.Heparanase enhances syndecan-1 shedding: a novel mechanism for stimulation of tumor growth and metastasis[J].J Bio Chem,2007,282(18):13326–13333.doi: 10.1074/jbc.M611259200.
[14]Mikami S,Ohashi K,Usui Y,et al.Loss of syndecan-1 and increased expression of heparanase in invasive esophageal carcinomas[J].Jpn J Cancer Res,2001,92(10):1062–1073.
[15]Masola V,Gambaro G,Tibaldi E,et al.Heparanase and syndecan-1 interplay orchestrates fibroblast growth factor-2-induced epithelialmesenchymal transition in renal tubular cells[J].J Biol Chem,2012,287(2):1478–1488.doi: 10.1074/jbc.M111.279836.
[16]Wang GB,Zhou XY,Wang XQ.Relationship between serum heparanase and microscopic venous invasion in patients with hepatocellular carcinoma[J].Am J Clin Pathol,2010,134(2):242–248.doi: 10.1309/AJCPPJM6VHG4LPJX.
[17]Xiong Z,Lü MH,Fan YH,et al.Downregulation of heparanase by RNA interference inhibits invasion and tumorigenesis of hepatocellular cancer cells in vitro and in vivo[J].Int J Oncol,2012,40(5):1601–1609.doi: 10.3892/ijo.2012.1338.
[18]Tang D,Zhang Q,Zhao S,et al.The expression and clinical significance of microRNA-1258 and heparanase in human breast cancer[J].Clin Biochem,2013,46(10/11):926–932.doi: 10.1016/j.clinbiochem.2013.01.027.
[19]Zheng L,Pu J,Jiang G,et al.Abnormal expression of early growth response 1 in gastric cancer: association with tumor invasion,metastasis and heparanase transcription[J].Pathol Int,2010,60(4):268–277.doi: 10.1111/j.1440–1827.2010.02512.x.
[20]Vlodavsky I,Beckhove P,Lerner I,et al.Significance of heparanase in cancer and inflammation[J].Cancer Microenviron,2012,5(2):115–132.doi: 10.1007/s12307–011–0082–7.
[21]Mogler C,Herold-Mende C,Dyckhoff G,et al.Heparanase expression in head and neck squamous cell carcinomas is associated with reduced proliferation and improved survival[J].Histopathology,2011,58(6):944–952.doi: 10.1111/j.1365–2559.2011.03834.x.
[22]Zeng C,Ke ZF,Luo WR,et al.Heparanase overexpression participates in tumor growth of cervical cancer in vitro and in vivo[J].Med Oncol,2013,30(1):403.doi: 10.1007/s12032–012–0403–9.
[23]Liu P,Gou M,Yi T,et al.The enhanced antitumor effects of biodegradable cationic heparin-polyethyleneimine nanogels delivering HSulf-1 gene combined with cisplatin on ovarian cancer[J].Int J Oncol,2012,41(4):1504–1512.doi: 10.3892/ijo.2012.1558.
[24]Lui NS,van Zante A,Rosen SD,et al.SULF2 expression by immunohistochemistry and overall survival in oesophageal cancer:a cohort study[J].BMJ Open,2012,2(6).pii: e001624.doi:10.1136/bmjopen-2012–001624.
[25]Ruan J,Trotter TN,Nan L,et al.Heparanase inhibits osteoblastogenesis and shifts bone marrow progenitor cell fate in myeloma bone disease[J].Bone,2013,57(1):10–17.doi: 10.1016/j.bone.2013.07.024.E
[26]Barash U,Zohar Y,Wildbaum G,et al.Heparanase enhances myeloma progression via CXCL10 downregulation[J].Leukemia,2014,28(11):2178–2187.doi: 10.1038/leu.2014.121.
[27]Zhang J,Yang JM,Wang HJ,et al.Synthesized multiple antigenic polypeptide vaccine based on B-cell epitopes of human heparanase could elicit a potent antimetastatic effect on human hepatocellular carcinoma in vivo[J].PLoS One,2013,8(1):e52940.doi: 10.1371/journal.pone.0052940.
[28]Waisberg J,Theodoro TR,Matos LL,et al.Immunohistochemical expression of heparanase isoforms and syndecan-1 proteins in colorectal adenomas[J].Eur J Histochem,2016,60(1):2590.doi:10.4081/ejh.2016.2590.
[29]Roucourt B,Meeussen S,Bao J,et al.Heparanase activates the syndecan-synteninALIX exosome pathway[J].Cell Research,2015,25(4):412–428.doi: 10.1038/cr.2015.29.
[30]Akbarshahi H,Axelsson JB,Said K,et al.TLR4 dependent heparan sulphate-induced pancreatic inflammatory response is IRF3-mediated[J].J Transl Med,2011,9:219.doi: 10.1186/1479–5876–9–219.
[31]Guo CH,Koo CY,Bay BH,et al.Comparison of the effects of differentially sulphated bovine kidney-and porcine intestinederived heparan sulphate on breast carcinoma cellular behaviour[J].Int J Oncol,2007,31(6):1415–1423.
[32]Heidari-Hamedani G,Vivès RR,Seffouh A,et al.syndecan-1 alters heparan sulfate composition and signaling pathways in malignant mesothelioma[J].Cell Signal,2015,27(10):2054–2067.doi:10.1016/j.cellsig.2015.07.017.
[33]Ilan N,Elkin M,Vlodavsky I.Regulation,function,and clinical significance of heparanase in cancer metastasis and angiogenesis[J].Int J Biochem Cell Biol,2006,38(12):2018–2039.
[34]Levy-Adam F,Ilan N,Vlodavsky I.Tumorigenic and adhesive propertities of heparanase[J].Semin Cancer Biol,2010,20(3):153–160.doi: 10.1016/j.semcancer.2010.06.005.
[35]Barash U,Cohen-Kaplan V,Doweck I,et al.Proteoglycans in health and diseases: New concepts for heparanase function in tumor progression and metastasis[J].FEBS J,2010,277(19):3890–3903.doi: 10.1111/j.1742–4658.2010.07799.x.
[36]Zetser A,Bashenko Y,Edovitsky E,et al.Heparanase induces vascular endothelial growth factor expression: correlation with p38 phosphorylation levels and SRC activation[J].Cancer Res,2006,66(3):1455–1463.DOI: 10.1158/0008–5472.CAN-05–1811
[37]Purushothaman A,Hurst DR,Pisano C,et al.Heparanase-mediated loss of nuclear syndecan-1 enhances histone acetyltransferase (HAT)activity to promote expression of genes that drive an aggressive tumor phenotype[J].J Biol Chem,2011,286(35):30377–30383.doi:10.1074/jbc.M111.254789.
[38]Mahtouk K,Hose D,Raynaud P,et al.Heparanase influences expression and shedding of syndecan-1,and its expression by the bone marrow environment is a bad prognostic factor in multiple myeloma[J].Blood,2007,109(11):4914–4923.doi: 10.1182/blood-2006–08–043232.
[39]Okawa T,Naomoto Y,Nobuhisa T,et al.Heparanase is involved in angiogenesis in esophageal cancer through induction of cyclooxygenase-2[J].Clin Cancer Res,2005,11(22):7995–8005.doi: 10.1158/1078–0432.CCR-05–1103.
[40]Cohen-Kaplan V,Naroditsky I,Zetser A,et al.Heparanase induces VEGF-C and facilitates tumor lymph angiogenesis[J].Int J Cancer,2008,123(11):2566–2573.doi: 10.1002/ijc.23898.
[41]Nadir Y,Brenner B,Zetser A,et al.Heparanase induces tissue factor expression in vascular endothelial and cancer cells[J].J Thromb Haemost 2006; 4(11):2443–2451.doi: 10.1111/j.1538–7836.2006.02212.x
[42]Yang Y,Ren Y,Ramani VC,et al.Heparanase enhances local and systemic osteolysis in multiple myeloma by upregulating the expression and secretion of RANKL[J].Cancer Res,2010,70(21):8329–8338.doi: 10.1158/0008–5472.CAN-10–2179.
[43]Ramani VC,Yang Y,Ren Y,et al.Heparanase plays a dual role in driving hepatocyte growth factor (HGF) signaling by enhancing HGF expression and activity[J].J Biol Chem,2011,286(8):6490–6499.doi: 10.1074/jbc.M110.183277.
[44]Cohen-Kaplan V,Jrbashyan J,Yanir Y,et al.Heparanase induces signal transducer and activator of transcription (STAT) protein phosphorylation: preclinical and clinical significance in head and neck cancer[J].J Biol Chem,2012,287(9):6668–6678.doi:10.1074/jbc.M111.271346.
[45]Masola V,Zaza G,Secchi MF,et al.Heparanase is a key player in renal fibrosis by regulating TGF-b expression and activity[J].Biochim Biophys Acta,2014,1843(9):2122–2128.doi: 10.1016/j.bbamcr.2014.06.005.