原发性肝癌(hepatocellular carcinoma,HCC)是危害生命健康的最主要的恶性肿瘤之一。肝细胞癌是最常见的肝癌类型,是世界范围内癌症死亡的第二大原因,特别是在HBV和HCV感染率较高的亚洲和非洲国家[1-3]。据2012年统计,全世界约有78万新发病例和74万死亡病例,我国占新发病例的50%,且呈明显的上升趋势[4]。而目前肝癌根治术是治疗HCC最有效的治疗手段之一[5]。但术后的高复发率仍是影响HCC患者预后的主要影响因素[6]。因此,本研究旨在探讨影响HCC术后早期复发的影响因素,从而一定程度上预测HCC早期复发。
1 资料与方法
1.1 一般资料
选取郑州大学第一附属医院2014年1月1日—2016年1月1日450例HCC行根治术的患者临床资料。入选标准:⑴ 首次发病且术前诊断为HCC;⑵ 术后切除标本病理明确诊断为HCC。排除标准:⑴ 术后病理诊断为胆管细胞癌及其他病理结果;⑵ 二次手术或术前放疗、化疗;⑶ 合并肺、脑等其他脏器转移;⑷ Child-Pugh分级C级的患者。
1.2 研究方法
通过嘉和病历系统收集患者的临床和病理学资料,包括术前一般资料(年龄、性别、BCLC分期、Child-Pugh分级、肿瘤数目、肿瘤最大直径、是否存在 HBV/HCV感染、术前血清AFP水平等),手术相关资料(肝门阻断时间、肿瘤分化程度、术中出血量、输血量)等,手术方式均采用开腹肝癌切除术。
1.3 随访
术后随访1~48个月,通过电话或住院资料查询记录患者术后有无复发及复发时间,随访时间至2018年1月1日。
1.4 统计学处理
应用SPSS 21.0专业统计学软件进行数据分析,所有的计数资料以例数(百分率)[n(%)]表示,采用χ2检验,采用Cox比例风险回归分析肝癌复发的危险因素。P<0.05为差异有统计学意义。
2 结 果
2.1 HCC患者术后复发情况
在450例行HCC切除术的患者中,经术后随访得到,复发患者共182例(40.4%),其中术后6个月内复发患者53例(29.1%),术后6个月至1年内复发患者62例(34.1%),术后1~2年内复发患者67例(36.8%)。
2.2 患者术后复发危险因素的单因素分析
结果显示:HCC患者手术切除后复发与肿瘤数目、肿瘤最大直径、血清AFP水平、肿瘤分化程度有关(均P<0.05),与年龄、性别、Child-Pugh分级、有无HBV/HCV感染、肝门阻断时间、术中出血量、输血量等方面,均无明显关系(均P>0.05)(表1)。
表1 HCC患者术后复发危险因素的单因素分析[n(%)]
Table1 Univariate analysis of risk factors for recurrence in HCC patients [n (%)]
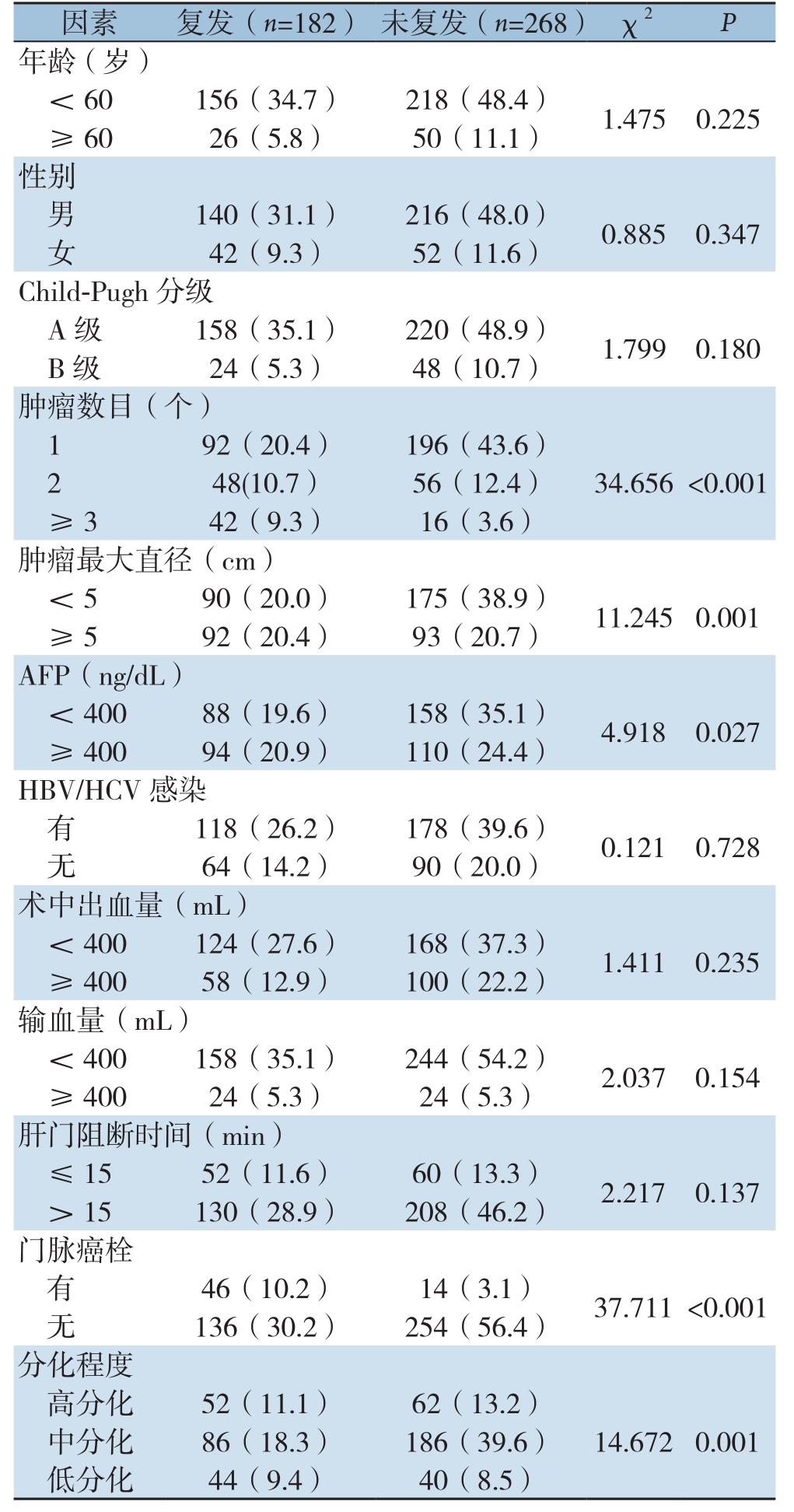
因素 复发(n=182) 未复发(n=268) χ2 P年龄(岁)<60156(34.7) 218(48.4) 1.4750.225≥ 6026(5.8) 50(11.1)性别男 140(31.1) 216(48.0) 0.8850.347女42(9.3) 52(11.6)Child-Pugh分级A级 158(35.1) 220(48.9) 1.7990.180 B级 24(5.3) 48(10.7)肿瘤数目(个)192(20.4) 196(43.6)248(10.7) 56(12.4) 34.656 <0.001≥ 342(9.3) 16(3.6)肿瘤最大直径(cm)<590(20.0) 175(38.9) 11.2450.001≥ 592(20.4) 93(20.7)AFP(ng/dL)<40088(19.6) 158(35.1) 4.9180.027≥ 40094(20.9) 110(24.4)HBV/HCV感染有 118(26.2) 178(39.6) 0.1210.728无64(14.2) 90(20.0)术中出血量(mL)<400124(27.6) 168(37.3) 1.4110.235≥ 40058(12.9) 100(22.2)输血量(mL)<400158(35.1) 244(54.2) 2.0370.154≥ 40024(5.3) 24(5.3)肝门阻断时间(min)≤1552(11.6) 60(13.3) 2.2170.137> 15130(28.9) 208(46.2)门脉癌栓有46(10.2) 14(3.1) 37.711 <0.001无 136(30.2) 254(56.4)分化程度高分化 52(11.1) 62(13.2)中分化 86(18.3) 186(39.6) 14.6720.001低分化 44(9.4) 40(8.5)
2.3 HCC术后复发危险因素的Cox比例风险回归分析
本研究以生存结局(HCC患者术后早期复发)和无复发生存时间作为因变量,以单因素筛选的有统计学意义的各相关因素作为自变量,进行多因素Cox比例风险回归模型分析(变量筛选方法Backward:LR,变量入选标准α=0.05,剔除标准为0.1)。结果:肿瘤数目、肿瘤最大直径、门脉有无癌栓、血清AFP水平、肿瘤分化程度是HCC患者术后早期复发的独立危险因素(均P<0.05)(表2)。
表2 HCC患者术后复发危险因素的Cox比例风险回归分析
Table2 Cox proportional risk regression analysis of risk factors for recurrence in patients with hepatocellular carcinoma after operation
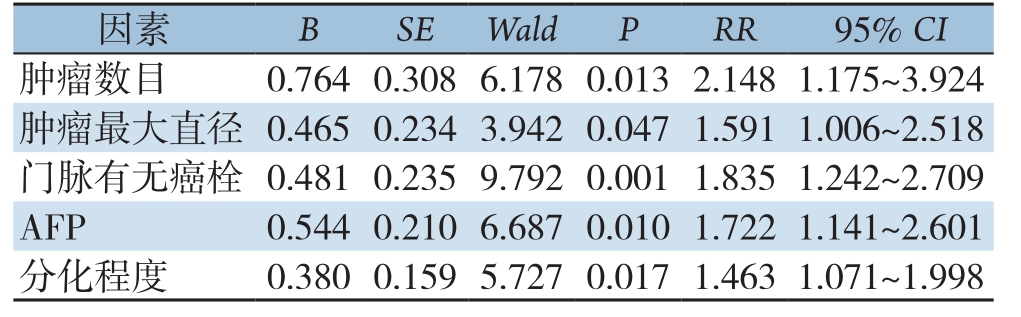
因素 B SE Wald P RR 95% CI肿瘤数目 0.7640.3086.1780.0132.1481.175~3.924肿瘤最大直径 0.4650.2343.9420.0471.5911.006~2.518门脉有无癌栓 0.4810.2359.7920.0011.8351.242~2.709 AFP 0.5440.2106.6870.0101.7221.141~2.601分化程度 0.3800.1595.7270.0171.4631.071~1.998
2.4 HCC术后复发危险因素的Cox比例风险回归分析模型
根据多因素Cox比例风险回归模型分析结果,拟合出HCC患者术后早期复发风险函数模型表达式为h(t)=h0exp(2.148X1+1.591X2+1.835X3+1.722X4+1.463X5)。该函数指数部分取值越大,则风险函数h(t)越大,则患者早期复发风险越高。预后指数(prognostic index,PI)=2.148X1+1.591X2+1.835X3+1.722X4+1.463X5。X1、X2、X3、X4、X5分别表示肿瘤数目、肿瘤最大直径、门脉有无癌栓、血清AFP水平、肿瘤分化程度;并对该预测模型进行似然比检验,模型差异有统计学意义(χ2=45.727,P<0.001)。
3 讨 论
在HCC患者诸多治疗方案中,手术切除术作为目前首选的有效治疗方案[7-8]。但是由于其复发率高,治疗性肝切除术后的长期预后并不令人满意。肝癌的复发一般考虑是由多中心癌变引起的,多中心癌变通常在肝切除术后较长时间发生,或转移通常较早发生[9-10]。术后高复发率也是早期复发(<2年)严重影响治疗效果,肿瘤早期复发是影响HCC患者根治性肝切除术后长期生存的重要因素[11-12]。早期复发主要取决于原发(切除)肿瘤的侵袭性特征,如肿瘤大小、血管侵袭性和较高的血清AFP水平。这些关联支持了早期复发可能源于原发肿瘤肝内转移的观点。相比之下,晚期复发主要与病因学和肝硬化背景有关,后者是肝癌发生的公认危险因素,并为新肿瘤的发展提供了肥沃的土壤[13]。但同时,晚期HCC的肿瘤复发和预后更可能由早期复发决定[14],故本研究专对可能造成术后早期复发的各种危险因素展开讨论。
本研究发现,在众多影响因素中原发性肝癌患者手术切除后早期复发与门脉有无癌栓、肿瘤数目、肿瘤最大直径、血清AFP水平、肿瘤分化程度有关。因此,通过建立函数模型h(t)=h0exp(2.148X1+1.591X2+1.835X3+1.722X4+1.463X5),能够在一定程度上预测早期复发的风险程度。首先血管侵犯,特别是微血管侵犯,是一个导致患者肿瘤分期、肿瘤进展更差的独立的预后因素[15]。而门静脉癌栓(portal vein tumor thrombus,PVTT)是肝癌患者常见的并发症,常提示病情进展、治疗困难、预后差。本研究中术后有门静脉癌栓的患者比没有门脉癌栓的患者更容易复发(P<0.05)。有研究[16]表示肝癌合并PVTT的发生率为44%~62.2%,中位生存期仅2.7个月。根据门静脉癌栓的不同位置,可分为四型:I0 型为显微镜下形成癌栓,I型为癌栓累及二级及二级以上门静脉分支(肝段门静脉及以上);II型累及一级门静脉分支(门静脉左右支);III型累及门静脉主干者为;IV型累及肠系膜上静脉或下腔静脉程氏分型[17]。由于门静脉分支充当肝癌的引流血管或肝癌通过静脉壁的直接侵袭,肿瘤细胞侵入肿瘤所输出的门静脉分支的腔内。PVTT的形成、肿瘤细胞侵入门静脉分支腔可再次导致I型PVTT,而直接血管壁侵入可导致II型或III型PVTT,导致HCC切除术后的复发[18-19]。门脉有无癌栓形成也可能与微血管癌栓形成肝内微转移灶,而术中未能完全清除而致术后复发有关[20]。
同样,肿瘤的数目和最大直径也是影响肝癌早期复发的主要影响因素之一。在本研究中,肿瘤数目越多,肿瘤最大直径越大,术后复发的可能性就越大(P<0.05)。尤其是直径>5 cm 的大肝癌,通常具有较高的侵袭性,有研究[21]显示肝癌直径若>5 cm,即使经病理检查发现手术切缘肿瘤细胞为阴性,但仍可见肿瘤的微卫星灶及镜下血管侵犯,发生肝内转移和大静脉侵犯的机会也较多。肿瘤数目多、肿瘤直径>5 cm复发风险高,也可能与肿瘤体积大,压迫或侵犯大血管及胆管等重要结构、肝硬化程度高、肝功能储备差、器官毗邻等因素有关[22]。Chan等[13]也指出,肿瘤大小是肝癌早期复发的关键参数。
肿瘤的分化程度一般代表肿瘤的生物学特性。本研究中分化程度越差,术后更容易复发(P<0.05)。分化程度越高的肿瘤组织,其恶性程度越低,侵袭性低,手术切除后复发率较低。反之,分化程度低的肿瘤侵袭性高,致使癌细胞增殖生长旺盛,易生长突破包膜,向周围组织浸润,早期易出现复发转移[23-24]。
AFP是一种重要的肿瘤标志物,是一种酸性糖蛋白。分子量为69000[25-26]。血药浓度在健康成人中AFP通常<25 ng/mL,如果血清AFP升高,可预测HCC、生殖细胞肿瘤、转移癌的高危性[27-28]。AFP是HCC的特异性标志物。本研究中血清AFP水平高的患者在复发患者总体中占51.6%,在总样本量中占20.9%(χ2=4.918,P=0.027)。安松林等[29]研究同样表明AFP是判断预后的重要因素,400 ng/mL可作为预测无复发生存的截点值。血清AFP越高,肝癌复发可能性就越大。Hu等[30]的研究也同样证实这一点。
本研究回顾性分析本医院450例HCC患者,在选择样本上存在一定程度的选择偏倚,故需要在进一步进行多中心、大样本的长期临床研究加以证实,并采用队列研究来验证本研究的函数模型。总而言之,门脉有无癌栓、肿瘤数目、最大直径、肿瘤分化程度可能是造成复发的独立危险因素,术前评估这些因素对预防术后复发有一定的指导意义。
[1] 刘绍平, 罗汉传, 林源, 等. 射频消融治疗复发性肝癌的应用价值[J]. 中国普通外科杂志, 2015, 24(1):23-26. doi:10.3978/j.issn.1005-6947.2015.01.005.Liu SP, Luo HC, Lin Y, et al. Application value of radiofrequency ablation in treatment of recurrent hepatocellular carcinoma[J].Chinese Journal of General Surgery, 2015, 24(1):23-26.doi:10.3978/j.issn.1005-6947.2015.01.005.
[2] 胡清雯, 钱国军. 冷循环微波消融结合TACE治疗肝癌的临床效果观察[J]. 中国普通外科杂志, 2018, 27(1):125-128. doi:10.3978/j.issn.1005-6947.2018.01.020.Hu QW, Qian GJ. Observation on clinical efficacy of cold circulation microwave ablation combined with TACE in treatment of hepatic cancer [J]. Chinese Journal of General Surgery, 2018,27(1):125-128. doi:10.3978/j.issn.1005-6947.2018.01.020.
[3] Liu MX, Jin L, Sun SJ, et al. Metabolic reprogramming by PCK1 promotes TCA cataplerosis, oxidative stress and apoptosis in liver cancer cells and suppresses hepatocellular carcinoma[J]. Oncogene,2018, 37(12):1637-1653. doi:10.1038/s41388-017-0070-6.
[4] 陈世发, 赵礼金. 肝癌发生发展机制的研究进展及其治疗现状[J]. 中国普通外科杂志, 2018, 27(7):910-923. doi:10.3978/j.issn.1005-6947.2018.07.016.Chen SF, Zhao LJ. Research progress on mechanisms for occurrence of liver cancer and its treatment status[J].Chinese Journal of General Surgery, 2018, 27(7):910-923. doi:10.3978/j.issn.1005-6947.2018.07.016.
[5] Capussotti L, Ferrero A, Viganò L, et al. Liver resection for HCC with cirrhosis:surgical perspectives out of EASL/AASLD guidelines[J]. Eur J Surg Oncol, 2009, 35(1):11-15. doi:10.1016/j.ejso.2007.06.005.
[6] Heimbach JK, Kulik LM, Finn RS,et al. AASLD guidelines for the treatment of hepatocellular carcinoma[J]. Hepatology, 2018,67(1):358-380. doi:10.1002/hep.29086.
[7] 张发鹏, 袁荣发, 张引, 等. 腹腔镜下射频消融治疗小肝癌的临床疗效分析[J]. 中国普通外科杂志, 2018, 27(1):35-41. doi:10.3978/j.issn.1005-6947.2018.01.006.Zhang FP, Yuan RF, Zhang Y, et al. Clinical efficacy of laparoscopic radiofrequency ablation in treatment of small primary hepatocellular carcinoma[J]. Chinese Journal of General Surgery, 2018, 27(1):35-41. doi:10.3978/j.issn.1005-6947.2018.01.006.
[8] 朱继领, 张克瑞. 射频消融与再手术治疗符合米兰标准的术后复发性肝癌的Meta分析[J]. 中国普通外科杂志, 2017, 26(7):838-846. doi:10.3978/j.issn.1005-6947.2017.07.005.Zhu JL, Zhang KR. Radiofrequency ablation versus surgical reresection for postoperative recurrent hepatocellular carcinoma within the Milan criteria:a Meta-analysis[J]. Chinese Journal of General Surgery, 2017, 26(7):838-846. doi:10.3978/j.issn.1005-6947.2017.07.005.
[9] Hayashi M, Shimizu T, Hirokawa F, et al. Clinicopathological risk factors for recurrence within one year after initial hepatectomy for hepatocellular carcinoma[J]. Am Surg, 2011, 77(5):572-578.
[10] Lu X, Zhao H, Yang H, et al. A prospective clinical study on early recurrence of hepatocellular carcinoma after hepatectomy[J]. J Surg Oncol, 2009, 100(6):488-493. doi:10.1002/jso.21354.
[11] Park JH, Koh KC, Choi MS, et al. Analysis of risk factors associated with early multinodular recurrences after hepatic resection for hepatocellular carcinoma[J]. Am J Surg, 2006, 192(1):29-33. doi:10.1016/j.amjsurg.2005.11.010.
[12] Lai CL, Wu PC, Lam KC, et al. Histologic prognostic indicators in hepatocellular carcinoma[J]. Cancer, 1979, 44(5):1677-1683.
[13] Chan AWH, Zhong J, Berhane S, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection[J]. J Hepatol, 2018, 69(6):1284-1293. doi:10.1016/j.jhep.2018.08.027.
[14] Audisio RA, Bombelli L, Lombardi L, et al. A clinico-pathologic study of clear-cell hepatocellular carcinoma[J]. Tumori, 1987,73(4):389-395.
[15] Zhang X, Li J, Shen F, et al. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma[J]. J Gastroenterol Hepatol,2018, 33(2):347-354. doi:10.1111/jgh.13843.
[16] Lin DX, Zhang QY, Li X, et al. An aggressive approach leads to improved survival in hepatocellular carcinoma patients with portal vein tumor thrombus[J]. J Cancer Res Clin Oncol, 2011,137(1):139-149. doi:10.1007/s00432-010-0868-x.
[17] 荚卫东, 刘文斌. 《肝细胞癌合并门静脉癌栓多学科诊治中国专家共识(2016年版)》解读[J]. 中国普通外科杂志, 2017,26(7):815-820. doi:10.3978/j.issn.1005-6947.2017.07.001.Jia WD, Liu WB. Interpretation of Chinese Expert Consensus on Multidisciplinary Diagnosis and Treatment of Hepatocellular Carcinoma with Portal Vein Tumor Thrombus (2016 edition)[J].Chinese Journal of General Surgery, 2017, 26(7):815-820. doi:10.3978/j.issn.1005-6947.2017.07.001.
[18] Chang IS, Shin SW, Cho SK, et al. Evolution of portal vein tumor thromboses in patients with hepatocellular carcinoma:CT findings and transition of serum tumor markers[J]. Clin Imaging, 2012,36(5):489-495. doi:10.1016/j.clinimag.2011.11.020.
[19] 周松强, 游燊, 邱福南, 等. 肝癌切除术中Glisson蒂横断与第一肝门阻断的应用比较[J]. 中国普通外科杂志, 2018, 27(1):129-134.doi:10.3978/j.issn.1005-6947.2018.01.021.Zhou SQ, You B, Qiu FN, et al. Application of Glisson's pedicle transection compared with occlusion of the first porta hepatis during hepatic cancer resection[J]. Chinese Journal of General Surgery,2018, 27(1):129-134. doi:10.3978/j.issn.1005-6947.2018.01.021.
[20] 汪超, 黄建钊. 肝细胞性肝癌术后复发的影响因素概况[J]. 中国民族民间医药, 2016, 25(11):31-33.Wang C, Huang JZ. Overview of factors for postoperative recurrence of hepatocellular carcinoma[J]. Chinese Journal of Ethnomedicine and Ethnopharmacy, 2016, 25(11):31-33.
[21] 张杰, 段伯焕, 朱功兵. 肝细胞癌患者外科手术根治术后生存分析[J]. 实用肝脏病杂志, 2017, 20(1):93-96. doi:10.3969/j.issn.1672-5069.2017.01.024.Zhang J, Duan BH, Zhu GB. Affecting factors of survival of patients with hepatocellular carcinoma after radical surgical resection[J].Journal of Practical Hepatology, 2017, 20(1):93-96. doi:10.3969/j.issn.1672-5069.2017.01.024.
[22] 安建立, 韩孝宇, 沙俊峰, 等. 肝动脉化疗栓塞序贯微波消融治疗初治大肝癌1年内复发的影响因素分析[J]. 现代肿瘤医学, 2018,26(18):2887-2892. doi:10.3969/j.issn.1672-4992.2018.18.016.An JL, Han XY, Sha JF, et al. Analysis of factors influencing 1 year's recurrence of primary large hepatocellu - lar car-cinoma treated with transcatheter arterial chemoembolization and sequ -ential micro-wave ablation[J]. Journal of Modern Oncology, 2018,26(18):2887-2892. doi:10.3969/j.issn.1672-4992.2018.18.016.
[23] 宋书红, 郭俊, 邹灿. 肝癌手术切除术后复发相关因素分析[J]. 实用肝脏病杂志, 2017, 20(2):203-206. doi:10.3969/j.issn.1672-5069.2017.02.019.Song SH, Guo J, Zou C. Factors influencing postoperative recurrence in patients with primary liver cancer after hepatectomy[J]. Journal of Practical Hepatology, 2017, 20(2):203-206. doi:10.3969/j.issn.1672-5069.2017.02.019.
[24] 黄金球, 彭民浩, 邹全庆, 等. 原发性肝癌切除术后早期复发高危因素分析 [J]. 中国实用外科杂志, 2009, 29(5):418-420.Huang JQ, Peng MH, Zou QQ, et al. Analysis of risk factors for early recurrence of primary hepatocellular carcinoma after radical hepatectomy[J]. Chinese Journal of Practical Surgery, 2009,29(5):418-420.
[25] Yuan Y, Yuan R, Chai Y,et al. A Reagentless Amperometric Immunosensor for Alpha-Fetoprotein Based on Gold Nanoparticles/TiO2 Colloids/Prussian Blue Modified Platinum Electrode[J].Electroanalysis, 2007, 19(13):1402-1410.
[26] Gao Q, Han J, Ma Z. Polyamidoamine dendrimers-capped carbon dots/Au nanocrystal nanocomposites and its application for electrochemical immunosensor[J]. Biosens Bioelectron, 2013,49:323-328. doi:10.1016/j.bios.2013.05.048.
[27] Di Bisceglie AM, Sterling RK, Chung RT, et al. Serum alphafetoprotein levels in patients with advanced hepatitis C:results from the HALT-C Trial[J]. J Hepatol, 2005, 43(3):434-441.
[28] Fu Z, Hao C, Fei X, et al. Flow-injection chemiluminescent immunoassay for alpha-fetoprotein based on epoxysilane modified glass microbeads[J]. J Immunol Methods, 2006, 312(1/2):61-67.doi:10.1016/j.jim.2006.02.006
[29] 安松林, 王黎明, 荣维淇, 等. 原发性肝细胞癌术前血清AFP的预后价值及与临床病理因素相关性分析[J]. 中华普通外科杂志, 2015, 30(3):189-193. doi:10.3760/cma.j.issn.1007-631X.2015.03.004.An SL, Wang LM, Rong WQ, et al. Prognostic significance of preoperative α-fetoprotein in hepatocellular carcinoma and its correlation with clinicopathological factors[J]. Zhong Hua Pu Tong Wai Ke Za Zhi, 2015, 30(3):189-193. doi:10.3760/cma.j.issn.1007-631X.2015.03.004.
[30] Hu L, Xue F, Li Y, et al. A long-term follow-up and comprehensive observation of risk and prognosis factors of recurrence and survival after resection of hepatocellular carcinoma[J]. Cell Biochem Biophys, 2014, 69(3):421-431. doi:10.1007/s12013-013-9813-3.
