移植静脉再狭窄的主要原因是血管内膜的过度增生,这是一个多因素参与的病理生理过程,具体的细胞和分子机制目前尚不清楚[1-2]。microRNA(miRNA)是一类小的非编码RNA,它们在转录后基因调控中起着至关重要的作用,在多种生物过程中调控基因表达,包括细胞分化,增殖,新陈代谢和炎症反应等[3]。miRNA与心血管疾病的发生发展也密切相关,多种心血管疾病中都有miRNA的异常表达[4-5]。近年来,以miRNA干预为基础的基因治疗方法已经成为心血管疾病研究中的一个新兴领域,并在相关的动物模型中取得了一定进展;比如miRNA介导的抗血管生成基因沉默可以有效促进缺血心肌局部新血管形成[6]。鉴于此,本研究通过构建大鼠自体静脉移植模型,采用高通量测序技术获取移植静脉的miRNA表达谱,并进行生物信息学分析,希望能为移植静脉再狭窄的防治工作提供更多线索。
1 材料与方法
1.1 主要实验仪器和试剂
苏木素-伊红(HE)染色试剂盒(武汉博士德公司);10%水合氯醛溶液(美国Sigma公司);4%多聚甲醛溶液(美国Sigma公司);TRIzol试剂(美国Invitrogen公司);mirVana RNA分离试剂盒(美国Applied Biosystems公司)。
1.2 实验动物及分组
无特定病原体的雄性Sprague-Dawley大鼠30只,体质量250~300 g,由上海西普尔-必凯实验动物有限公司提供,许可证号码:SCXK(沪)2018-0006,合格证编号:20180006004335。实验动物的操作及饲养均符合《实验动物管理条例》相关规定,并通过郑州大学动物实验伦理委员会审查和批准。实验大鼠被随机分为3组:对照组、术后3 d组(POD 3组)、术后14 d组(POD 14组),每组10只。
1.3 实验方法
1.3.1 动物模型的制作 术前禁食12 h。10%水合氯醛(0.4 mL/100 g)行腹腔注射麻醉,待麻醉满意后,四肢固定,仰卧于恒温垫上,维持肛温在36.5~37.5 ℃之间。备皮,碘伏消毒手术区域,铺巾。取左侧胸锁乳突肌前缘切口,显露并分离左侧颈外静脉截取中间静脉约0.8 cm备用。取腹正中切口约3~4 cm长,游离、显露腹主动脉。无创血管夹将其阻断,精细剪离断腹主动脉,使用10-0无损伤缝线,采用间断缝合的方法,将颈外静脉移植到肾下腹主动脉上并缝合后腹膜。还纳胃和肠管,6-0线逐层缝合腹膜、肌肉、皮下组织和皮肤。关腹完毕,碘伏消毒伤口。术后动物房分笼饲养,勤换垫料,注意观察。分别于术后3、14 d取材,用于后续实验。对照组大鼠仅分离出左侧颈外静脉,不做移植处理。
1.3.2 总RNA的抽提与测序 使用mirVana RNA试剂盒从移植血管中提取总RNA,Nanodrop ND-2000对总RNA进行定量处理,Agilent Bioanalyzer 2100评估RNA质量。合格样本交由上海欧易生物医学科技有限公司进行测序工作。
1.3.3 miRNA的表达与差异分析 对原始数据进行标准化处理,通过火山图评估所检测到的miRNA整体分布情况,然后对miRNA进行非监督层次聚类分析。最后按照|fold change|>2且P<0.05的条件筛选出差异表达的基因。
1.3.4 靶基因的功能分析 基因本体论(GO)富集分析,即对该物种差异表达的miRNA靶基因进行功能注释和归类,将该物种所有基因作为背景列表,差异表达的miRNA靶基因作为从背景列表中筛选出来的候选列表,利用超几何分布检验计算代表GO功能集在差异miRNA靶基因列表中是否显著富集的P值,并检验纠正。京都基因与基因组百科全书(KEGG)信号通路富集分析,KEGG是有关细胞通路的公共数据库,利用KEGG数据库对差异 miRNA靶基因进行细胞通路分析,并计算每条通路条目中差异miRNA靶基因富集的显著性。计算所得小的P值表示差异miRNA靶基因在该通路中出现了富集。
1.4 统计学处理
采用 SPSS 17.0 统计软件进行统计学分析,数据以均数±标准差(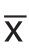 ±s)表示,均数比较采用配对t检验及单因素方差分析,P<0.05表示差异具有统计学意义。
±s)表示,均数比较采用配对t检验及单因素方差分析,P<0.05表示差异具有统计学意义。
2 结果
2.1 造模结果
HE染色结果显示,对照组大鼠颈外静脉血管壁较薄,内膜仅为单层的内皮细胞,无内膜增生;POD 3组内膜未见明显增生;POD 14组可见明显的内膜增生,血管壁开始增厚(图1)。
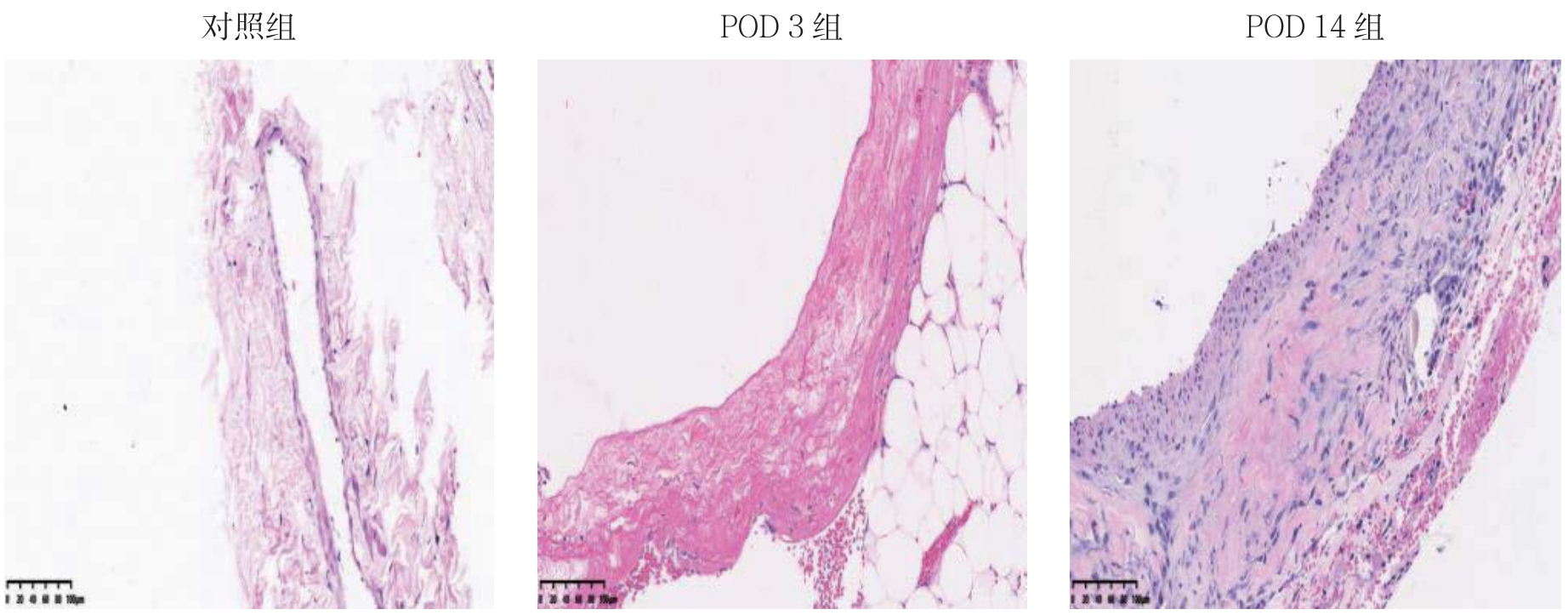
图1 各组静脉内膜变化情况(HE×200)
Figure 1 Changes in venous intima of each group(HE×200)
2.2 miRNA的表达与差异分析结果
POD 14组与对照组比较,明显上调的miRNA数目为183,明显下调的miRNA数目为82;POD 14组与POD 3组比较,明显上调miRNA数目为94,明显下调的miRNA数目为64。绘制火山图了解差异表达的miRNA整体分布情况(图2)。对差异表达的miRNA进行非监督层次聚类分析,同一类样本能通过聚类出现在同一个簇中,而聚在同一个簇中的miRNA可能具有相似的生物学功能(图3)。
2.3 靶基因功能分析结果
GO富集分析筛选出了生物学过程(BP)、细胞组分(CC)、分子功能(MF)3种分类中富集基因数目排在前10位的GO条目,结果提示富集的基因主要参与了对DNA转录过程的调节、对细胞间信号转导的调控和对蛋白质磷酸化过程的调节(图4)。绘制topGO有向无环图,直观展示靶基因富集的GO节点及其层级关系;分支代表的包含关系,从上至下所定义的功能描述范围越来越具体,对GO的3大分类(CC,BP,MF)的每一类都取富集程度最高的前10位作为无环图的主节点,用矩形表示,并通过包含关系将相关联的GO条目一起展示,颜色的深浅表示富集程度,颜色越深代表富集程度越高(图5)。KEGG信号通路分析筛选结果见图6(图中每个小点对应一个通路,颜色按红,橙,黄,绿,蓝,靛,紫对应P值从小到大排序,P值越小,颜色越趋向于红色,点越大表示该通路中基因个数越多),结果显示,最有可能参与移植静脉内膜增生过程的信号通路有MAPK信号通路、cAMP信号通路、Wnt信号通路、紧密连接、cGMP-PKG信号 通路。
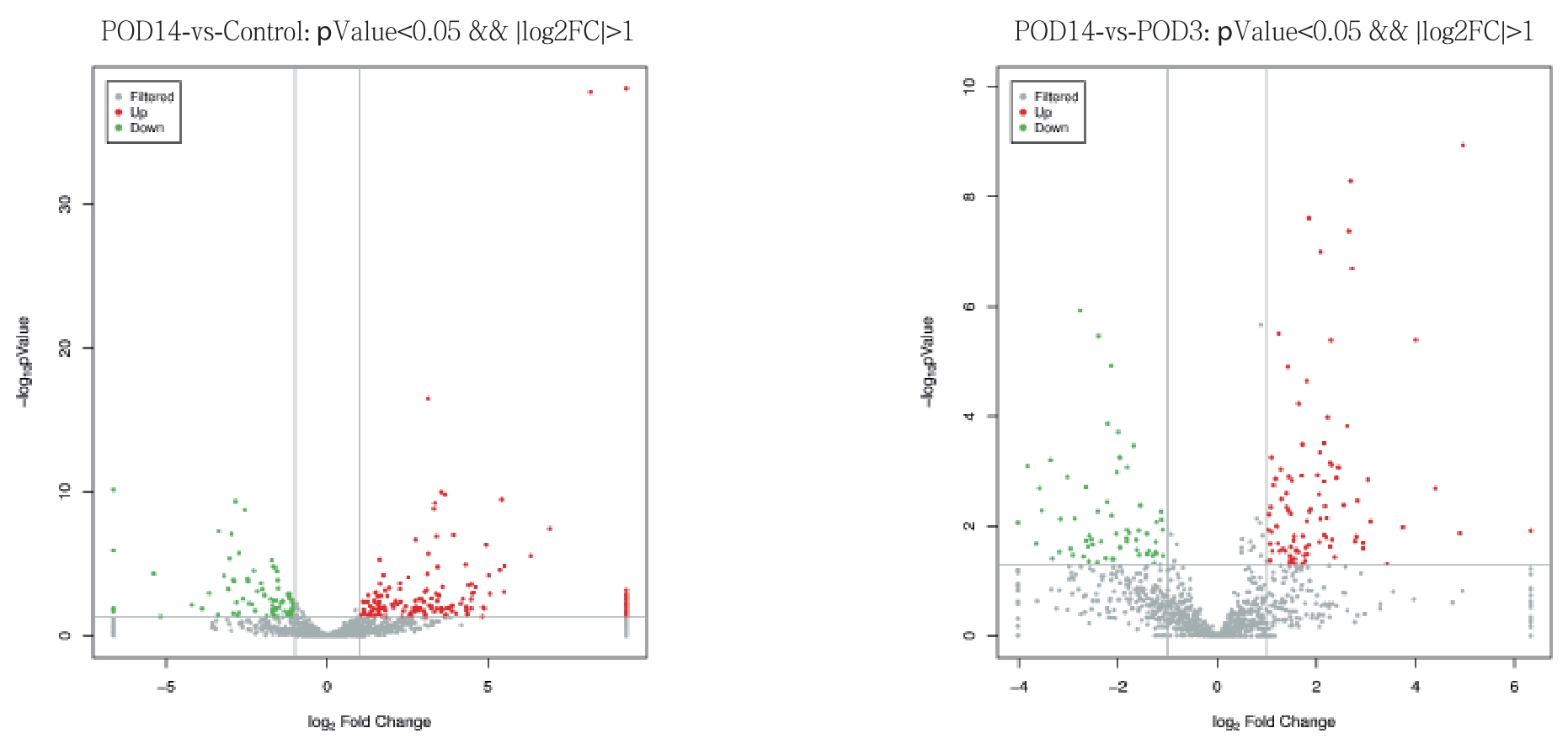
图2 反应各组间差异表达miRNA整体分布情况的火山图
Figure 2 Volcano plots representing the general distributions of the diff erentially expressed miRNAs

图3 差异性表达miRNA的聚类分析(红色表示高表达,蓝色表示低表达)
Figure 3 Cluster analysis of the diff erentially expressed miRNAs(red color representing high expression,and blue color representing low expression)
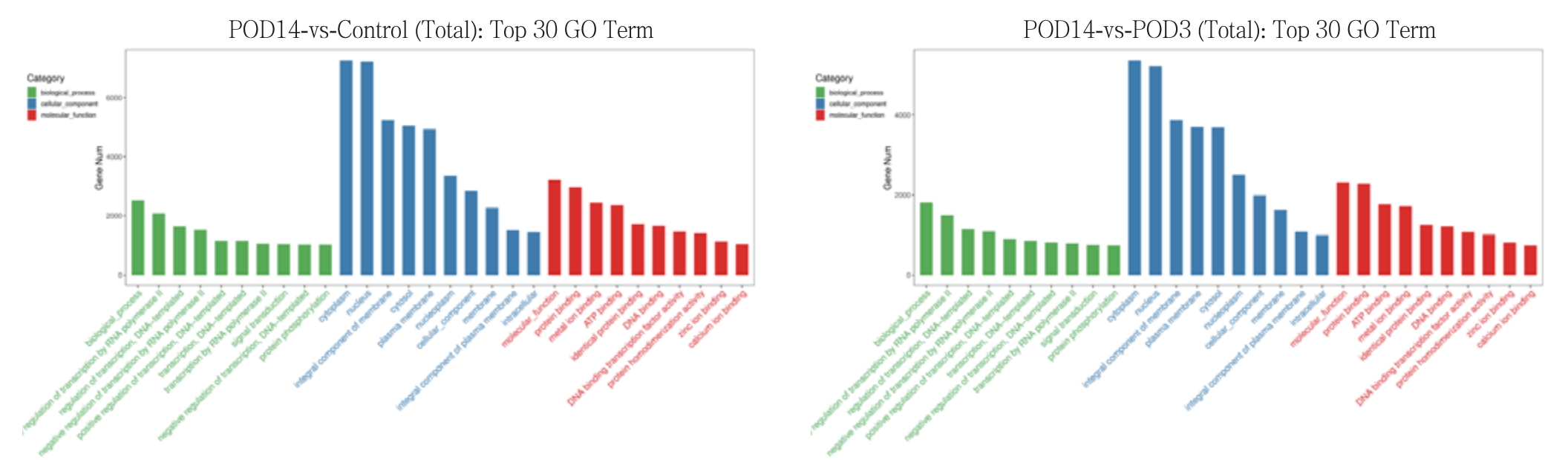
图4 差异表达miRNA的靶基因的GO富集分析
Figure 4 GO enrichment analysis of the target genes of the diff erentially expressed miRNAs
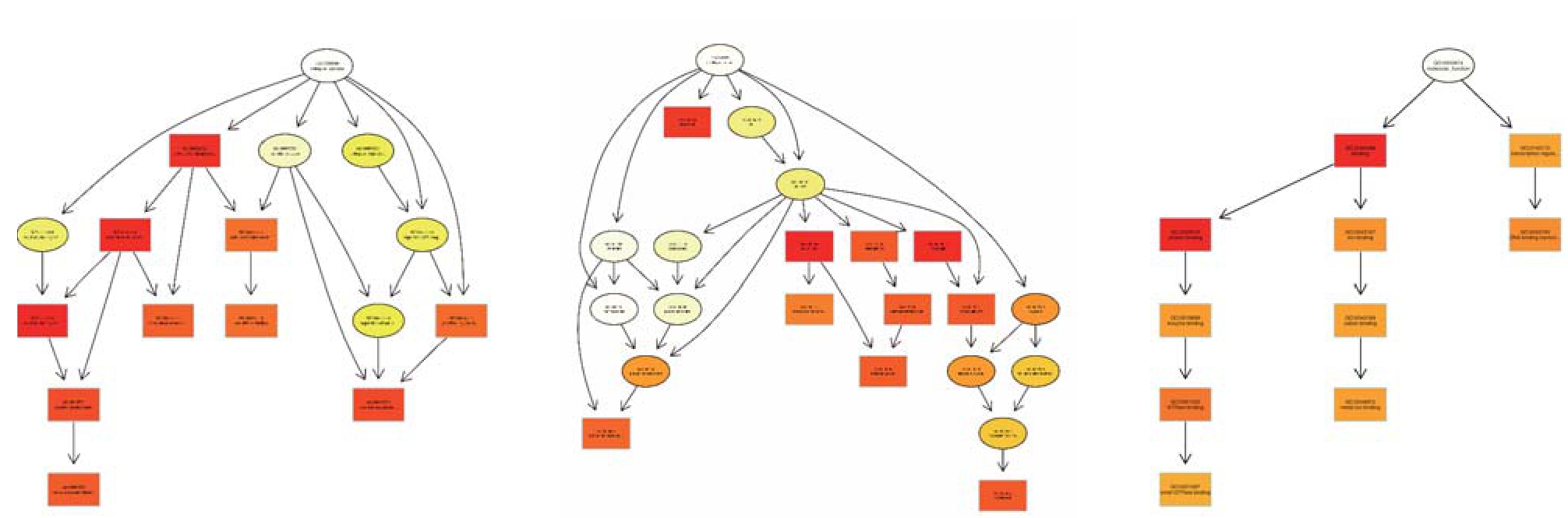
图5 部分差异表达miRNA靶基因topGO有向无环图
Figure 5 The topGO-directed acyclic graph for some target genes of the diff erentially expressed miRNAs

图6 差异表达miRNA靶基因进行KEGG通路分析
Figure 6 KEGG pathway analysis of the target genes of the diff erentially expressed miRNAs
3 讨论
miRNA常通过与靶基因发生特异性结合发挥调控作用[7-8]。一种miRNA可调节多种靶基因,而一种靶基因也可分别被多种miRNA所结合[9-10]。多项研究[11-13]表明miRNA是心血管疾病重要的调控因子。有报道[14]称miRNA参与了颈动脉损伤后的内膜增生过程,过表达miR-146a可促进血管紧张素Ⅱ诱导的血管平滑肌细胞增殖,改善颈动脉损伤后的内膜增生。Zhang等[15]认为miR-451可以通过MAPK通路缓解血管平滑肌细胞损伤从而保护新生内膜。这些证据提示miRNA能够参与了血管内膜增生过程。实际上,在防治移植静脉再狭窄的研究中,基于miRNA干预的基因治疗方法已经被证明是可行有效的[16]。Wen等[17]开发了一种可以搭载TMC-g-PEG-VAPG/miRNA-145复合物的新型功能性电纺膜,实现了miRNA-145的定向传递,能够持久抑制血管平滑肌细胞过度增殖和血管内膜增生。Cao等[18]通过慢病毒转染技术实现了let-7a在大鼠移植静脉中的高表达从而抑制大鼠静脉移植术后内膜的过度增生。miR-221在调控血管平滑肌细胞增殖和表型转化中发挥重要作用,腺病毒介导的miR-221基因治疗可以抑制静脉移植物新生内膜增生[19]。
大鼠静脉移植模型是研究移植静脉再狭窄机制重要工具。移植静脉在术后的早期阶段以细胞坏死凋亡、基质分解为主;术后第3天,血管中的平滑肌细胞开始由收缩型向合成型转变。术后第7~28天是细胞增殖高峰时期,这个阶段血管平滑肌细胞发生增殖和迁移、细胞外基质合成,静脉的内膜厚度增加[20-21]。本研究选取术后3、14 d两个时间点进行测序,能够较好地反映移植静脉在内膜增生过程中的miRNA表达变化情况。
POD 14组与对照组比较,显著上调的miRNA数目为183,显著下调的miRNA数目为82;POD 14组与POD 3组相比较,显著上调miRNA数目为94,显著下调的miRNA数目为64。其中部分差异表达的miRNA已被证实与血管平滑肌细胞的增殖迁移过程相关[22-25]。有研究报道miRNA-92a可促进血管平滑肌细胞的增殖和迁移[26-27];本研究还发现某些未报道过的miRNA在大鼠的移植血管中表达升高,参与了移植静脉内膜增生过程,但具体的调控机制还需要进一步研究。通过对miRNA靶基因进行功能分析,我们发现富集的信号通路有MAPK信号通路、cAMP信号通路、Wnt信号通路、紧密连接结构、cGMP-PKG信号通路等,这显示出miRNA调控作用的广泛性和机制的复杂性。
总之,本研究通过测序技术检测了大鼠移植静脉miRNA差异表达情况,这将为防治移植静脉再狭窄的研究提供更多有价值的信息。
[1]Wallitt EJ,Jevon M,Hornick PI.Therapeutics of vein graft intimal hyperplasia:100 years on[J].Ann Thorac Surg,2007,84(1):317-323.doi:10.1016/j.athoracsur.2007.02.035.
[2]严力.大隐静脉源性血管平滑肌细胞表型转化的研究进展[J].中国普通外科杂志,2018,27(6):768-775.doi:10.3978/j.issn.1005-6947.2018.06.017.Yan L.Research progress in phenotypic transformation of vascular smooth muscle cells from great saphenous vein[J].Chinese Journal of General Surgery,2018,27(6):768-775.doi:10.3978/j.issn.1005-6947.2018.06.017.
[3]He L,Hannon GJ.MicroRNAs:small RNAs with a big role in gene regulation[J].Nat Rev Genet,2004,5(7):522-531.doi:10.1038/nrg1379.
[4]McManus DD,Freedman JE.MicroRNAs in platelet function and cardiovascular disease[J].Nat Rev Cardiol,2015,12(12):711-717.doi:10.1038/nrcardio.2015.101.
[5]焦通,姚野,孙庆峰,等.ADAM10、ADAM17的作用和miRNA的调节在腹主动脉瘤发病机制中的研究进展[J].中国普通外科杂志,2016,25(6):908-912.doi:10.3978/j.issn.1005-6947.2016.06.023.Jiao T,Yao Y,Sun QF,et al.Actions of ADAM10 and ADAM17 and regulatory role of miRNAs in pathogenesis of abdominal aortic aneurysm[J].Chinese Journal of General Surgery,2016,25(6):908-912.doi:10.3978/j.issn.1005-6947.2016.06.023.
[6]Arif M,Pandey R,Alam P,et al.MicroRNA-210-mediated proliferation,survival,and angiogenesis promote cardiac repair post myocardial infarction in rodents[J].J Mol Med(Berl),2017,95(12):1369-1385.doi:10.1007/s00109-017-1591-8.
[7]Bernardo BC,Ooi JY,Lin RC,et al.miRNA therapeutics:a new class of drugs with potential therapeutic applications in the heart[J].Future Med Chem,2015,7(13):1771-1792.doi:10.4155/fmc.15.107.
[8]Mal C,Aftabuddin M,Kundu S.IIKmTA:Inter and Intra Kingdom miRNA-Target Analyzer[J].Interdiscip Sci,2018,10(3):538-543.doi:10.1007/s12539-018-0291-6.
[9]Fabian MR,Sonenberg N,Filipowicz W.Regulation of mRNA translation and stability by microRNAs[J].Annu Rev Biochem,2010,79:351-379.doi:10.1146/annurev-biochem-060308-103103.
[10]Fan Y,Xia J.miRNet-Functional Analysis and Visual Exploration of miRNA-Target Interactions in a Network Context[J].Methods Mol Biol,2018,1819:215-233.doi:10.1007/978-1-4939-8618-7_10.
[11]Bartel DP.MicroRNAs:genomics,biogenesis,mechanism,and function[J].Cell,2004,116(2):281-297.doi:10.1016/s0092-8674(04)00045-5
[12]Jiao T,Yao Y,Zhang B,et al.Role of MicroRNA-103a Targeting ADAM10 in Abdominal Aortic Aneurysm[J].Biomed Res Int,2017,2017:9645874.doi:10.1155/2017/9645874.
[13]Li F,Wang J,Zhu Y,et al.SphK1/S1P Mediates PDGF-Induced Pulmonary Arterial Smooth Muscle Cell Proliferation via miR-21/BMPRII/Id1 Signaling Pathway[J].Cell Physiol Biochem,2018,51(1):487-500.doi:10.1159/000495243.
[14]Zhao XS,Zheng B,Wen Y,et al.Salvianolic acid B inhibits Ang II-induced VSMC proliferation in vitro and intimal hyperplasia in vivo by downregulating miR-146a expression[J].Phytomedicine,2019,58:152754.doi:10.1159/000495243.
[15]Zhang W,Liu D,Han X,et al.MicroRNA-451 inhibits vascular smooth muscle cell migration and intimal hyperplasia after vascular injury via Ywhaz/p38 MAPK pathway[J].Exp Cell Res,2019,379(2):214-224.doi:10.1016/j.phymed.2018.11.014.
[16]魏立春.自体移植静脉再狭窄基因水平的研究进展[J].中国普通外科杂志,2013,22(6):766-770.DOI:10.7659/j.issn.1005-6947.2013.06.021.Wei LC.Stenosis of autogenous vein graft:processes at the gene level[J].Chinese Journal of General Surgery,2013,22(6):766-770.DOI:10.7659/j.issn.1005-6947.2013.06.021.
[17]Wen M,Zhou F,Cui C,et al.Performance of TMC-g-PEG-VAPG/miRNA-145 complexes in electrospun membranes for targetregulating vascular SMCs[J].Colloids Surf B Biointerfaces,2019,182:110369.doi:10.1016/j.colsurfb.2019.110369.
[18]Cao H,Hu X,Zhang Q,et al.Upregulation of let-7a inhibits vascular smooth muscle cell proliferation in vitro and in vein graft intimal hyperplasia in rats[J].J Surg Res,2014,192(1):223-233.doi:10.1016/j.jss.2014.05.045.
[19]Wang XW,He XJ,Lee KC,et al.MicroRNA-221 sponge therapy attenuates neointimal hyperplasia and improves blood flows in vein grafts[J].Int J Cardiol,2016,208:79-86.doi:10.1016/j.ijcard.2016.01.006.
[20]Wang Q,Wan L,Liu L,et al.Role of the mTOR Signalling Pathway in Experimental Rabbit Vein Grafts[J].Heart Lung Circ,2016,25(11):1124-1132.doi:10.1016/j.hlc.2016.03.009.
[21]Borin TF,Miyakawa AA,Cardoso L,et al.Apoptosis,cell proliferation and modulation of cyclin-dependent kinase inhibitor p21(cip1)in vascular remodelling during vein arterialization in the rat[J].Int J Exp Pathol,2009,90(3):328-337.doi:10.1111/j.1365-2613.2009.00648.x.
[22]Wang D,Gao B,Yue J,et al.Exosomes from mesenchymal stem cells expressing miR-125b inhibit neointimal hyperplasia via myosin IE[J].J Cell Mol Med,2019,23(2):1528-1540.doi:10.1111/jcmm.14060.
[23]Chen Z,Wang M,Huang K,et al.MicroRNA-125b Affects Vascular Smooth Muscle Cell Function by Targeting Serum Response Factor[J].Cell Physiol Biochem,2018,46(4):1566-1580.doi:10.1159/000489203.
[24]Cao C,Zhang H,Zhao L,et al.miR-125b targets DNMT3b and mediates p53 DNA methylation involving in the vascular smooth muscle cells proliferation induced by homocysteine[J].Exp Cell Res,2016,347(1):95-104.doi:10.1016/j.yexcr.2016.07.007.
[25]Qian DH,Gao P,Feng H,et al.Down-regulation of mir-542-3p promotes neointimal formation in the aging rat[J].Vascul Pharmacol,2015,72:118-129.doi:10.1016/j.vph.2015.05.010.
[26]Wang J,Zhang C,Li C,et al.MicroRNA-92a promotes vascular smooth muscle cell proliferation and migration through the ROCK/MLCK signalling pathway[J].J Cell Mol Med,2019,23(5):3696-3710.doi:10.1111/jcmm.14274.
[27]Liu P,Su J,Song X,et al.miR-92a regulates the expression levels of matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 3 via sirtuin 1 signaling in hydrogen peroxide-induced vascular smooth muscle cells[J].Mol Med Rep,2018,17(1):1041-1048.doi:10.3892/mmr.2017.7937.
