皮革胃是胃癌的一种特殊类型,癌组织呈弥漫性侵犯胃壁,造成胃壁变厚僵硬、胃腔缩小,其在确诊时多属晚期,常伴有淋巴结转移、脉管浸润,其发病率占所有胃癌的3%~19%[1]。皮革胃患者的预后较非皮革胃差,术后5年生存率仅为3%~10%[2-4]。AJCC-TNM分期系统是目前全球公认的胃癌分期系统[5]。然而,最近有研究[6]显示,AJCC-TNM系统对皮革胃患者长期预后的区分度较低,因此,Blackham等[2]研究认为AJCCTNM分期系统可能并不适用于皮革胃患者。列线图是一种结合多个影响预后的危险因素、可以较准确评估总体生存或无复发生存的工具,具有形象直观、简单实用等特点[7]。目前,列线图用于评估肺癌、结肠癌、胃癌术后长期预后已取得了满意的效果[6, 8-9],但尚未见应用于皮革胃的报道。本文旨在建立一个准确预测皮革胃患者术后生存率的列线图模型。
1 资料与方法
1.1 一般资料
2008年12月—2014年9月共有274例患者在福建医科大学附属协和医院胃外科诊断为皮革胃,排除临床资料不全的患者(6例)、失访患者(12例)、IV期患者(53例)后,最终203例行R0切除的患者被纳入本研究。全组患者的临床病理资料包括:年龄、性别、病理类型、分化程度、浸润深度、转移淋巴结数目、受检淋巴结数目、辅助化疗、生存数据等。全组患者均未行术前辅助化疗或者放疗。术前临床分期根据胃镜、腹部CT、全腹彩超等方法综合判断。皮革胃诊断:患者胃镜下表现为肿瘤弥漫浸润生长、正常胃形态消失、呈现“皮革样”状胃;CT或胃镜提示肿瘤>10 cm、且累及胃两部及以上者。诊断最后须经病理确诊。根据日本第14版《胃癌处理规约》选择胃切除范围并进行胃周淋巴结的清扫[5]。结合术中探查情况,保证切缘阴性的R0切除下,部分患者行胃大部切除术。术后肿瘤病理和分期根据第七版AJCCTNM分期系统标准。根据NCCN指南,建议术后病理分期为II、III期皮革胃患者行术后辅助化疗,化疗周期为6个月,化疗用药以5-FU为主的单药或联合化疗。全组203例患者中,115例在我中心接受术后辅助化疗;88例未在我中心接受辅助化疗,其中有47例患者在当地医院接受辅助化疗(具体化疗方案、用药及周期不明),41例患者由于经济、家属意愿等原因未接受术后辅助化疗。
1.2 随访
本研究的研究终点为3年总体生存率,随访终点是2017年9月,或者患者死亡。总体生存时间按照从手术所在月份到最后1次随访、任何原因死亡或研究终点所在月份之间的时间计算。术后随访,2年内每3个月1次,2年后每半年至1年1次,随访内容包括症状体征采集、实验室检查、影像学检查、胃镜。
1.3 研究方法与统计学处理
计量资料用均值±标准差( ±s)表示,分类变量用χ2检验或Fisher's精确检验。采用Cox比例风险模型对术后总体生存的危险因素进行单因素、多因素分析,结果以HR值和相应的95%的置信区间(CI)表示。应用R软件(3.2.0版本)描绘列线图:安装R软件,加载安装MASS、foreign、splines、rms、Hmisc工具包,将指令代码(略)输入R软件操作界面。术后总体生存率根据Kaplan-Meier法计算,Log-rank检验分析组间存活率的差异。线性χ2、阳性似然比(positive likelihood ratio,PLR)、赤池信息量准则(Akaike information criterion,AIC)值用于比较列线图和第7版AJCC-TNM分期系统的预测精度。线性χ2值越高,显示模型异质性辨别越佳;PLR值越高,显示模型同质性越稳定;AIC值越低,显示模型预后分层越准确[10-11]。线性χ2、PLR由SPSS 18.0统计软件计算,AIC值由R软件计算。当P<0.05时认为差异有统计学意义。所有数据应用SPSS 18.0统计软件进行统计学处理。
±s)表示,分类变量用χ2检验或Fisher's精确检验。采用Cox比例风险模型对术后总体生存的危险因素进行单因素、多因素分析,结果以HR值和相应的95%的置信区间(CI)表示。应用R软件(3.2.0版本)描绘列线图:安装R软件,加载安装MASS、foreign、splines、rms、Hmisc工具包,将指令代码(略)输入R软件操作界面。术后总体生存率根据Kaplan-Meier法计算,Log-rank检验分析组间存活率的差异。线性χ2、阳性似然比(positive likelihood ratio,PLR)、赤池信息量准则(Akaike information criterion,AIC)值用于比较列线图和第7版AJCC-TNM分期系统的预测精度。线性χ2值越高,显示模型异质性辨别越佳;PLR值越高,显示模型同质性越稳定;AIC值越低,显示模型预后分层越准确[10-11]。线性χ2、PLR由SPSS 18.0统计软件计算,AIC值由R软件计算。当P<0.05时认为差异有统计学意义。所有数据应用SPSS 18.0统计软件进行统计学处理。
2 结 果
2.1 一般临床病理资料
203例皮革胃患者的一般临床病理资料如表1示。全组患者中,男152例(74.8%),女51例(25.2%),平均年龄60.3(21~89)岁。肿瘤平均大小9.6(5.3~20.0)cm,全组患者平均BMI为21.5(9.6~31.2)kg/m2。160例患者行全胃切除术,43例行远侧胃大部切除术。pT4期和pN3期的皮革胃患者分别占全体患者的80%、66.8%。
2.2 影响患者预后的单因素及多因素分析
中位随访时间为38(2~111)个月。全组患者的3年总体生存率为31.2%,5年总体生存率为18.7%。全组患者根据第7版AJCC-TNM分期系统的生存曲线见图1。单因素分析提示,年龄>65岁、BMI<18.5 kg/m2、手术方式、肿瘤分化程度、T分期、N分期,淋巴结转移率和麻醉学分级是影响皮革胃患者预后的危险因素(均P<0.05)。对以上危险因素进行多因素分析,BMI<18.5 kg/m2(HR=1.65,95% CI=1.15~2.36,P=0.006)、肿瘤分化程度(HR=1.71,95% CI=1.14~2.57,P=0.042)、T分期(HR=2.46,95% CI= 0.57~10.59,P=0.032)、N分期(HR=2.14,95% CI=1.11~4.14,P=0.032)、ASA分级(HR=1.59,95% CI=0.89~2.28,P=0.016)是预后的独立危险因素(表2)。
表1 患者的临床病理资料
Table 1 Clinical and pathological characteristics of the patients
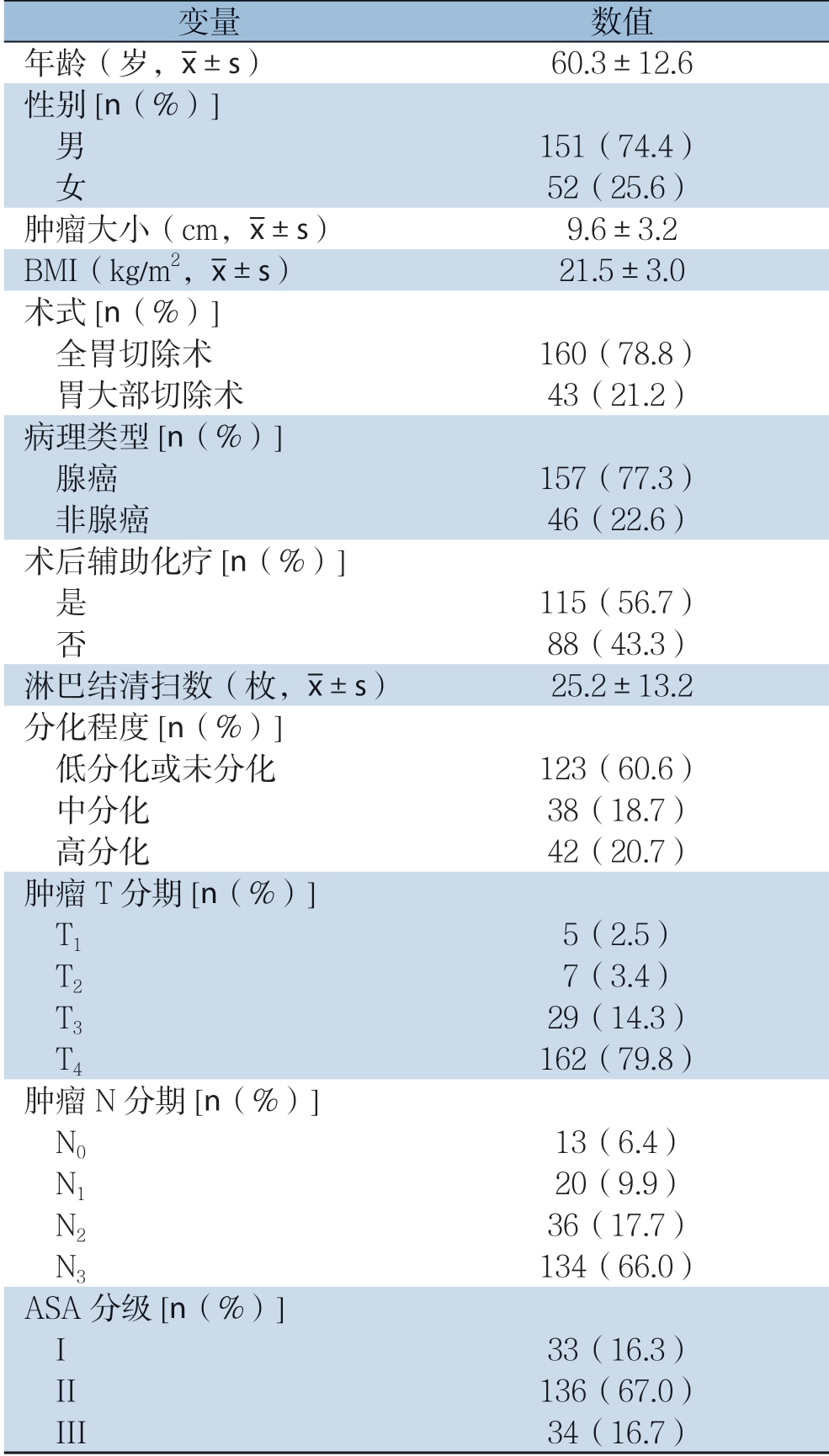
变量 数值年龄(岁, ±s) 60.3±12.6性别[n(%)]男 151(74.4) 女 52(25.6)肿瘤大小(cm,
±s) 60.3±12.6性别[n(%)]男 151(74.4) 女 52(25.6)肿瘤大小(cm, ±s) 9.6±3.2 BMI(kg/m2,
±s) 9.6±3.2 BMI(kg/m2, ±s) 21.5±3.0术式[n(%)]全胃切除术 160(78.8) 胃大部切除术 43(21.2)病理类型[n(%)]腺癌 157(77.3) 非腺癌 46(22.6)术后辅助化疗[n(%)]是 115(56.7) 否 88(43.3)淋巴结清扫数(枚,
±s) 21.5±3.0术式[n(%)]全胃切除术 160(78.8) 胃大部切除术 43(21.2)病理类型[n(%)]腺癌 157(77.3) 非腺癌 46(22.6)术后辅助化疗[n(%)]是 115(56.7) 否 88(43.3)淋巴结清扫数(枚, ±s) 25.2±13.2分化程度[n(%)]低分化或未分化 123(60.6) 中分化 38(18.7) 高分化 42(20.7)肿瘤T分期[n(%)]T1 5(2.5) T2 7(3.4) T3 29(14.3) T4 162(79.8)肿瘤N分期[n(%)]N0 13(6.4) N1 20(9.9) N2 36(17.7) N3 134(66.0)ASA分级[n(%)]I 33(16.3) II 136(67.0) III 34(16.7)
±s) 25.2±13.2分化程度[n(%)]低分化或未分化 123(60.6) 中分化 38(18.7) 高分化 42(20.7)肿瘤T分期[n(%)]T1 5(2.5) T2 7(3.4) T3 29(14.3) T4 162(79.8)肿瘤N分期[n(%)]N0 13(6.4) N1 20(9.9) N2 36(17.7) N3 134(66.0)ASA分级[n(%)]I 33(16.3) II 136(67.0) III 34(16.7)
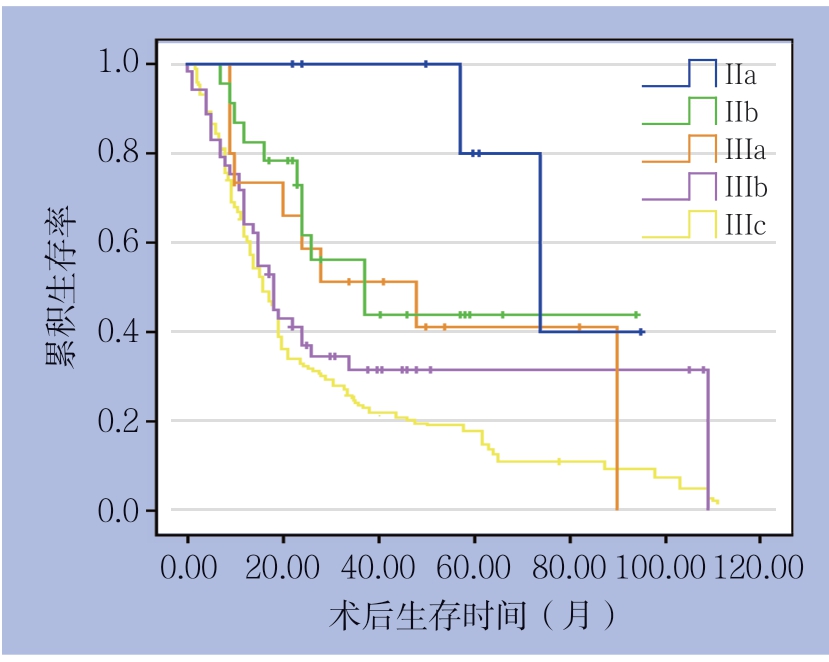
图1 根据第7版AJCC-TNM分期的患者生存曲线
Figure 1 Survival curves of the patients according 7th AJCCTNM staging system
表2 患者术后总体生存的单因素和多因素分析
Table 2 Univariate and multivariate analyses of the overall survivals of the patients
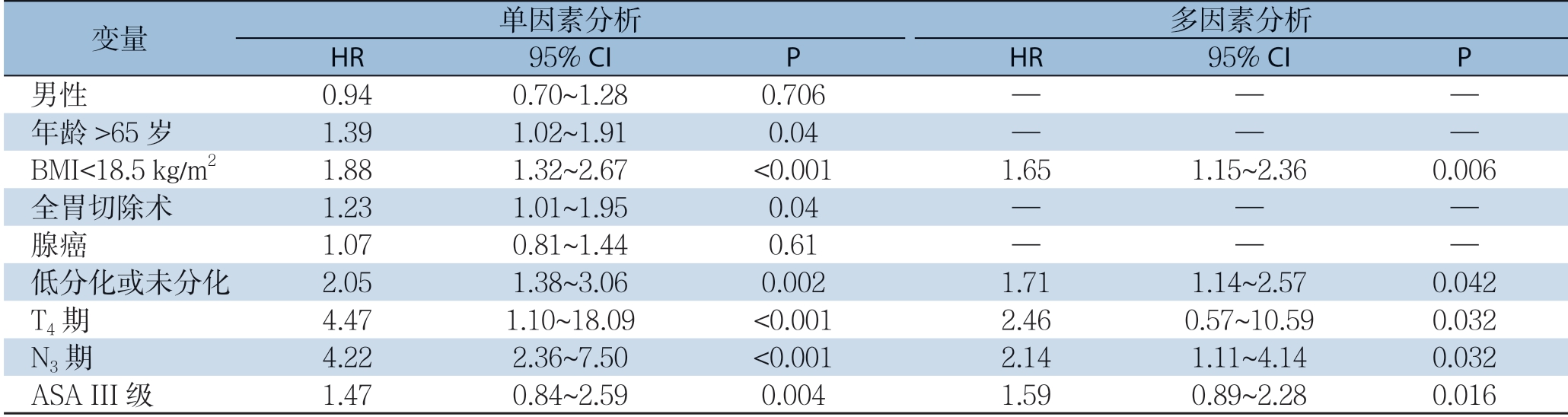
变量 单因素分析 多因素分析HR 95% CI P HR 95% CI P 男性 0.94 0.70~1.28 0.706 — — —年龄>65岁 1.39 1.02~1.91 0.04 — — —BMI<18.5 kg/m2 1.88 1.32~2.67 <0.001 1.65 1.15~2.36 0.006全胃切除术 1.23 1.01~1.95 0.04 — — —腺癌 1.07 0.81~1.44 0.61 — — —低分化或未分化 2.05 1.38~3.06 0.002 1.71 1.14~2.57 0.042 T4期 4.47 1.10~18.09 <0.001 2.46 0.57~10.59 0.032 N3期 4.22 2.36~7.50 <0.001 2.14 1.11~4.14 0.032 ASA III级 1.47 0.84~2.59 0.004 1.59 0.89~2.28 0.016
2.3 列线图预测模型的建立
根据多因素分析的结果,纳入BMI、肿瘤分化程度、T分期、N分期、麻醉学分级5个独立危险因素,建立了评估预后的列线图(图2)。根据列线图得分高低进行三等分,全组患者分为3组:低危组(<8分)、中危组(8~<16分)和高危组(≥16分),分析结果显示,3组患者的生存差异具有统计学意义(P<0.001)(图3)。
2.4 列线图预测模型与第7版AJCC-TNM分期系统的比较
列线图预测模型与第7版AJCC-TNM分期系统的线性χ2、PLR、AIC值分别为68.99 vs. 58.58、70.18 vs. 58.36、1473.38 vs. 1485.04,列线图在线性χ2、PLR、AIC值方面均优于AJCC-TNM分期。
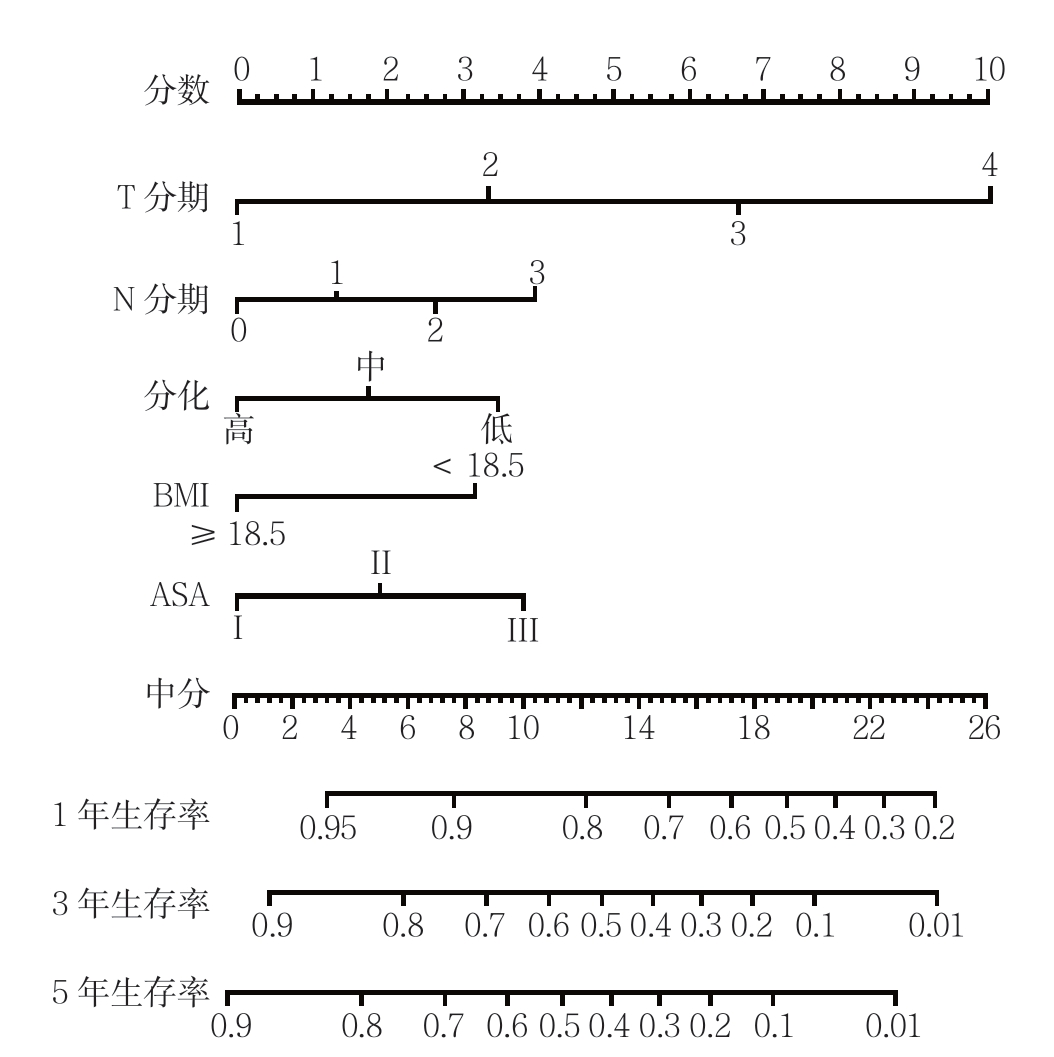
图2 根据多因素结果建立的列线图预测模型
Figure 2 Nomogram predictive model constructed according to result of multivariate analysis
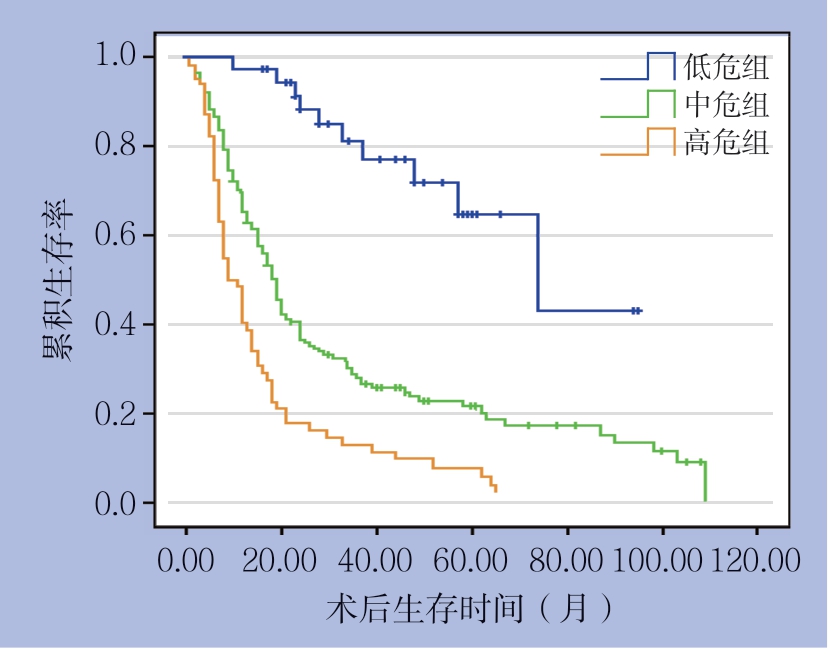
图3 不同危险分组患者的生存曲线
Figure 3 Survival curves of all patients in different risk groups
3 讨 论
作为胃癌的特殊类型,皮革胃进展迅速,在确诊时多为晚期,肿瘤组织多浸润至浆膜下层和浆膜层,且淋巴结转移数>7枚的占大多数,因此,皮革胃预后较一般胃腺癌更差[1, 12-13]。皮革胃好发于年轻女性、易发生腹腔或远处转移、不易进行根治性手术及复发率高等区别于一般胃癌的特点[14],本研究人群中,肿瘤T3+T4期占总人群的95.4%;淋巴结N3期占66.8%,这与文献报道的基本相符。
目前,国内外关于皮革胃预后的研究甚少。Otsuji等[15]对150例皮革胃患者分析发现,相较于非皮革胃患者,皮革胃患者的5年生存率仅10.5%。来自韩国的另一项研究发现,肿瘤是否R0切除、肿瘤T分期、肿瘤N分期、肿瘤细胞分化程度是皮革胃患者的独立预后因素,相同TNM分期的部分患者预后存在差异[14]。由此可见,除了经典的肿瘤T分期、肿瘤N分期和远侧转移外,可能存在其他影响皮革胃患者预后的危险因素。本研究发现,低BMI和高ASA评分也是皮革胃患者的独立危险因素,这可能与低BMI和高ASA评分影响患者全身营养、身体状态有关[16-17]。
目前的指南并未将皮革胃与一般胃癌进行区分。然而,由于皮革胃的生物学特性不同于一般胃腺癌,因此,目前的AJCC-TNM分期系统可能并不适用于评估皮革胃患者的预后情况。结合肿瘤的病理特征和其他独立危险因素建立的列线图模型用于评估癌症术后生存率,在食管癌、胃食管结合部癌、直肠癌、乳腺癌等其他癌症已有报道,其有效性也已得到证实[18-22]。列线图具有直观、简便、准确的特点,适合用于预后的个体化预测,预测结果计算简便,根据患者临床病理资料,选择相应的节点分值相加,以总分对应概率即可获得1、3、5年的生存率[7, 23]。近年来,有研究[24-26]建立了评估胃癌术后生存率的列线图。Kim等[6]建立的列线图预测胃癌患者的疾病特异性生存和总体生存的准确性优于TNM分期系统。Novotny等[27]提出结合年龄、肿瘤大小、Lauren分型等因素建立的列线图其对胃癌患者预后的区分度优于TNM分期系统。但针对皮革胃患者术后生存率的列线图模型还未见报道。本研究首次建立了评估皮革胃患者术后生存率的列线图模型。相较于目前报道的评估胃癌术后预后的列线图模型,除了肿瘤T分期、肿瘤N分期、肿瘤分化程度这3个预后因素外,本研究中的列线图模型还纳入了ASA评分和BMI等能够较全面体现皮革胃患者身体状况的预后因素。统计分析的结果表明,运用列线图得分对患者进行危险分组发现,高危组、中危组和低危组患者存率的差异显著(P<0.001)。进一步研究发现列线图模型的线性卡方、阳性似然比高于第7版AJCC-TNM分期系统且AIC值低于第7版AJCC-TNM分期系统。由此可见,列线图预测皮革胃患者个体化生存的精确度和区分度均优于第7版AJCC-TNM分期系统。因此,这一简单的预后模型可能有助于临床医生准确、全面评估皮革胃患者术后的预后情况。
本研究的局限性和不足之处有:本研究系单中心回顾性临床研究,难免存在一定的偏倚,期待今后多中心前瞻性大宗病例的验证;研究仅纳入R0切除的病例,缺少对R1切除、姑息性切除等的预后分析;未进一步详细分析化疗方案和用药周期等对皮革胃患者术后预后的影响。尽管如此,为了更好地评估皮革胃患者术后的生存率,本研究建立的预测模型具有相当的临床实用性和指导意义。
总之,联合BMI、肿瘤T分期、肿瘤N分期、分化程度、ASA评分建立的列线图模型能准确预测皮革胃患者术后的总体生存率,其预测准确度优于第7版AJCC-TNM分期系统。
[1]Issam Beyrouti M, Beyrouti R, Ben Amar M, et al. Linitis Plastica[J]. Presse Med, 2007, 12(Pt 2):1782-1786. doi: 10.1016/j.lpm.2007.03.004.
[2]Blackham AU, Swords DS, Levine EA, et al. Is Linitis Plastica a Contraindication for Surgical Resection: A Multi-Institution Study of the U.S. Gastric Cancer Collaborative[J]. Ann Surg Oncol, 2016, 23(4):1203-1211. doi: 10.1245/s10434-015-4947-8.
[3]Salvon-Harman JC, Cady B, Nikulasson S, et al. Shifting Proportions of Gastric Adenocarcinomas[J]. Arch Surg, 1994, 129(4):381-388.
[4]Park JC, Lee YC, Kim JH, et al. Clinicopathological aspects and prognostic value with respect to age: an analysis of 3,362 consecutive gastric cancer patients[J]. J Surg Oncol, 2009, 99(7):395-401. doi: 10.1002/jso.21281.
[5]Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4)[J]. Gastric Cancer, 2017, 20(1):1-19. doi: 10.1007/s10120-016-0622-4.
[6]Kim Y, Spolverato G, Ejaz A, et al. A nomogram to predict overall survival and disease-free survival after curative resection of gastric adenocarcinoma[J]. Ann Surg Oncol, 2015, 22(6):1828-1835. doi: 10.1245/s10434-014-4230-4.
[7]Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis[J]. J Clin Oncol, 2008, 26(8):1364-1370. doi: 10.1200/JCO.2007.12.9791.
[8]Young KA, Efiong E, Dove JT, et al. External Validation of a Survival Nomogram for Non-Small Cell Lung Cancer Using the National Cancer Database[J]. Ann Surg Oncol, 2017, 24(6):1459-1464. doi: 10.1245/s10434-017-5795-5.
[9]Simkens GA, van Oudheusden TR, Nieboer D, et al. Development of a Prognostic Nomogram for Patients with Peritoneally Metastasized Colorectal Cancer Treated with Cytoreductive Surgery and HIPEC[J]. Ann Surg Oncol, 2016, 23(13):4214-4221. doi: 10.1245/s10434-016-5211-6.
[10]Yoon HM, Ryu KW, Nam BH, et al. Is the New Seventh AJCC/UICC Staging System Appropriate for Patients with Gastric Cancer?[J]. J Am Coll Surg, 2012, 214(1):88-96. doi: 10.1016/j.jamcollsurg.2011.09.018.
[11]Sun Z, Wang ZN, Zhu Z, et al. Evaluation of the Seventh Edition of American Joint Committee On Cancer TNM Staging System for Gastric Cancer: Results From a Chinese Monoinstitutional Study[J]. Ann Surg Oncol, 2012, 19(6):1918-1927. doi: 10.1245/s10434-011-2206-1.
[12]Mastoraki A, Papanikolaou IS, Sakorafas G, et al. Facing the challenge of managing linitis plastica--review of the literature[J]. Hepatogastroenterology, 2009, 56(96):1773-1778.
[13]Wang J, Dang P, Raut CP, et al. Comparison of a lymph node ratiobased staging system with the 7th AJCC system for gastric cancer: analysis of 18,043 patients from the SEER databas[J]. Ann Surg, 2012, 255(3):478-485. doi: 10.1097/SLA.0b013e31824857e2.
[14]An JY, Kang TH, Choi MG, et al. Borrmann type IV: an independent prognostic factor for survival in gastric cancer[J]. J Gastrointest Surg, 2008, 12(8):1364-1369. doi: 10.1007/s11605-008-0516-9.
[15]Otsuji E, Yamaguchi T, Sawai K, et al. Regional lymph node metastasis as a predictor of peritoneal carcinomatosis in patients with Borrmann type IV gastric carcinoma[J]. Am J Gastroenterol, 1999, 94(2):434-437. doi: 10.1111/j.1572-0241.1999.873_b.x.
[16]Lee JH, Park B, Joo J, et al. Body mass index and mortality in patients with gastric cancer: a large cohort study[J]. Gastric Cancer, 2018, 21(6):913-924. doi: 10.1007/s10120-018-0818-x.
[17]Xu BB, Lu J, Zheng ZF, et al. Comparison of short-term and long-term efficacy of laparoscopic and open gastrectomy in highrisk patients with gastric cancer: a propensity score-matching analysis[J]. Surg Endosc, 2018, doi: 10.1007/s00464-018-6268-z. [Epub ahead of print]
[18]Zhou Z, Zhang H, Xu Z, et al. Nomogram predicted survival of patients with adenocarcinoma of esophagogastric junction[J]. World J Surg Oncol, 2015, 13:197. doi: 10.1186/s12957-015-0613-7.
[19]Kattan MW, Gönen M, Jarnagin WR, et al. A nomogram for predicting disease-specific survival after hepatic resection for metastatic colorectal cancer[J]. Ann Surg, 2008, 247(2):282-287. doi: 10.1097/SLA.0b013e31815ed67b.
[20]Matsuda N, Hayashi N, Ohde S, et al. A nomogram for predicting locoregional recurrence in primary breast cancer patients who received breast-conserving surgery after neoadjuvant chemotherapy[J]. J Surg Oncol, 2014, 109(8):764-769. doi: 10.1002/jso.23586.
[21]Chen K, Su F, Jacobs LK. A Nomogram to Predict the Benefit of Radiation Therapy After Breast-Conserving Surgery in Elderly Patients with Stage I & ER-Negative, or Stage II/III Disease[J]. Ann Surg Oncol, 2015, 22(11):3497-3503. doi: 10.1245/s10434-015-4393-7.
[22]Lagarde SM, Reitsma JB, de Castro SM, et al. Prognostic nomogram for patients undergoing oesophagectomy for adenocarcinoma of the oesophagus or gastro-oesophageal junction[J]. Br J Surg, 2007, 94(11):1361-1368. doi: 10.1002/bjs.5832.
[23]Han DS, Suh YS, Kong SH, et al. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer[J]. J Clin Oncol, 2012, 30(31):3834-3840. doi: 10.1200/JCO.2012.41.8343.
[24]Liu X, Wu Z, Lin E, et al. Systemic Prognostic Score and Nomogram Based On Inflammatory, Nutritional and Tumor Markers Predict Cancer-Specific Survival in Stage II-III Gastric Cancer Patients with Adjuvant Chemotherapy[J]. Clin Nutr, 2018, pii: S0261-5614(18)31217-31222. doi: 10.1016/j.clnu.2018.07.015.
[25]Zheng ZF, Lu J, Wang W, et al. Development and External Validation of a Simplified Nomogram Predicting Individual Survival After R0 Resection for Gastric Cancer: An International, Multicenter Study[J]. Ann Surg Oncol, 2018, 25(8):2383-2390. doi: 10.1245/s10434-018-6551-1.
[26]Wang W, Sun Z, Deng JY, et al. A novel nomogram individually predicting disease-specific survival after D2 gastrectomy for advanced gastric cancer[J]. Cancer Commun (Lond), 2018, 38(1):23. doi: 10.1186/s40880-018-0293-0.
[27]Novotny AR, Schuhmacher C, Busch R, et al. Predicting Individual Survival After Gastric Cancer Resection: Validation of a U.S.-Derived Nomogram at a Single High-Volume Center in Europe[J]. Ann Surg, 2006, 243(1):74-81.
