肿瘤相关成纤维细胞(cancer-associated fibroblasts,CAFs)旁分泌多种生长因子、蛋白水解酶等促进乳腺癌细胞生长、侵袭、转移及多种临床药物耐受[1-4]。值得注意的是,乳腺癌微环境中约80%的正常成纤维细胞被激活为CAFs,是肿瘤组织中最主要的成分之一[5]。G蛋白偶联雌激素受体(G protein-coupled estrogen receptor,GPER)被公认为第三种独立作用的雌激素受体,在多种正常细胞与恶性肿瘤细胞系中均证实17-β雌二醇(E2)及GPER特异性激动剂G1均可活化GPER并介导下游雌激素效应[6-13],是预测乳腺癌患者临床预后、内分泌治疗获得性耐药及靶向治疗的有效生物学指标。研究团队在前期实验中发现,GPER介导CAFs中芳香化酶基因CYP19A1与糖酵解基因PDK4高转录活性,可能是促进肿瘤组织中局部雌激素水平增加与酸性微环境的关键因素,从而在乳腺癌细胞增殖、转移及药物耐受中扮演始动作用[11-13],GPER或是介导CAFs与肿瘤细胞之间“交叉对话”进而促进乳腺癌进展的重要媒介。
成纤维细胞生长因子(fibroblast growth factors,FGFs)是一类能调节细胞增殖、分化的多肽家族,在机体许多组织和器官内均有不同程度的分布。现有研究[14-16]表明,成纤维细胞生长因子2(FGF2)的过量表达与肿瘤发生发展密切相关,不仅参与肿瘤血管新生,还间接调控肿瘤细胞的生长及侵袭转移能力。然而,乳腺癌微环境CAFs中GPER介导的生长因子调节作用研究较少,其与FGF2的调控关系及下游生物学作用更是研究“盲点”。因此,本研究拟利用E2、G1及FGF2中和抗体[17]等小分子化合物首次探讨共培养条件下乳腺癌微环境CAFs中细胞浆GPER活化对FGF2的表达调控作用及其旁分泌对肿瘤细胞生长的影响。
1 材料与方法
1.1 实验材料与仪器
MCF-7细胞系(ER+)和MDA-MB-468细胞系(ER-)购自中国科学院上海细胞库,研究团队前期已构建体外稳定培养的永生化CAFs细胞株[11-13],高糖DMEM培养基(Gibco公司),南美胎牛血清(Gibco公司)。E2与GPER特异性激动剂G1(Sigma公司),FGF2中和抗体(Millipore公司),DAPI与FITC标记山羊抗兔IgG抗体(北京中杉金桥公司),FGF2 ELISA试剂盒(Rapidbio公司),总RNA提取试剂盒、逆转录试剂盒(TaKaRa公司),荧光定量PCR试剂盒(BioTeKe公司),GPER抗体(Abcam公司),CCK-8试剂盒(碧云天公司)。流式细胞仪(FACS Callur),CO2培养箱(Thermo scientific),多功能酶标仪(Sunrise),MiniOpticon实时PCR仪和稳压DNA电泳仪(BioRad公司)。
1.2 方法
1.2.1 细胞培养 MCF-7、MDA-MB-468 及 CAFs细胞均培养于10%胎牛血清的高糖DMEM培养基中,37℃、5% CO2培养箱内生长,隔日换液,待细胞密度生长至90%左右时用0.25%胰酶消化离心后传代。
1.2.2 乳腺肿瘤细胞条件培养基的获取 乳腺癌MCF-7与MDA-MB-468细胞生长于10% FBS的高糖DMEM培养基,待细胞密度生长至约70%~80%,将培养液更换为1% FBS的培养基继续培养30 h后,将培养基收集后离心,其上清液即为肿瘤细胞条件培养基(conditioned medium,CM)[13]。
1.2.3 CAFs与乳腺癌细胞共培养模型 根据实验需求选取以下方式:⑴收集肿瘤细胞CM处理CAFs细胞 36 h;⑵Transwell小室法共培养 24 h,CAFs细胞种于上室,肿瘤细胞种于下室,细胞比例为3:1。分别得到CAFs+MCF-7与CAFs+MDAMB-468共培养细胞模型[13]。
1.2.4 GPER核输出信号(NES)序列突变 依托上海吉码制药技术有限公司对GPER NES序列:YFINLAVADLILV的核心区域LIL进行突变,得到突变型YFINLAVADAAAV的质粒,课题组已在前期研究中用脂质体2000转染等方法获得稳定筛选的GPER NES序列突变型细胞株CAFs(M)[13]。
1.2.5 细胞免疫荧光检测CAFs细胞中GPER表达定位 取单独培养及共培养条件下对数期生长的野生型CAFs与突变型CAFs(M)细胞胰酶消化离心重悬后以2×105/mL接种于底部放有小玻片的24孔板中,置于细胞孵箱中培养,待细胞生长状态及密度合适后,取出24孔板用PBS洗3次,加入4%多聚甲醛200 μL固定20min,PBS洗3次,10%山羊血清37℃封闭30 min,加入GPER抗体(1:100稀释)30~40 μL,4℃孵育过夜,PBS洗3次,暗室中加入FITC荧光二抗(1:200),37℃60min,PBS洗3次,加入DAPI(1:10)染核5min,PBS洗3次,每次 5 min,封片后,倒置荧光显微镜下拍照。
1.2.6 荧光定量PCR检测CAFs细胞中FGF2 mRNA表达量 CAFs细胞单独培养或取不同的共培养细胞模型,待CAFs细胞生长至70%~80%后,更换无血清培养基饥饿24 h。分别加入100 nmol/L E2与100 nmol/L G1 处理 CAFs细胞12 h(药物处理浓度参考研究团队前期研究[13]),各组调整DMSO至一致,最后分别得到对照组、E2处理组、G1处理组,处理相应时间后立即终止药物处理,用TRIzol试剂提取各组CAFs细胞中总RNA,逆转录为cDNA。逆转录及PCR反应均按照TaKaRa试剂盒及荧光定量PCR试剂盒说明进行操作。FGF2上游引物序列为5'-AGC CAG GTA ACG GTT AGC AC-3',下游引物序列为5'-GGA GAA GAG CGA CCC TCA C-3';以 β-actin为内参对照,上游引物序列为5'-AGC GAG CAT CCC CCA AAG TT-3',下游引物序列为5'-GGG CAC GAA GGC TCA TCA TT-3'。实验重复 3 次。
1.2.7 ELISA 法检测 CAFs细胞上清中 FGF2的分泌量 实验分组同1.2.6,待CAFs细胞生长至80%~90%后,更换无血清培养基饥饿24 h。分别加入100nmol/LE2与100nmol/LG1处理CAFs细胞24 h,各组调整DMSO至一致,最后分别得到对照组、E2处理组、G1处理组,处理相应时间后立即终止药物处理,收集细胞上清液,保存于-80℃冰箱,按ELISA试剂盒操作说明书检测各组样本中FGF2的含量。实验重复3次。
1.2.8 流式细胞术检测乳腺癌细胞周期 取对数生长期MCF-7、MDA-MB-468与CAFs细胞胰酶消化离心重悬后,接种于6孔板及Transwell小室中,用10%胎牛血清的无酚红高糖DMEM培养基培养至肿瘤细胞密度达到约50%,移去培养基,PBS洗2次,加入无血清无酚红DMEM培养基饥饿24 h,使各组细胞周期同步于G0/G1期。分别加入100 nmol/L E2、100 nmol/L G1与2 μg/mL FGF2中和抗体处理上室中CAFs细胞24 h,各组调整DMSO至一致,24 h后收获下室中乳腺癌细胞,70%乙醇固定,置于4℃冰箱过夜,PI染色避光室温孵育30 min,流式细胞仪检测肿瘤细胞中各细胞周期中DNA的含量。实验重复3次。
1.2.9 CCK8法检测乳腺癌细胞增殖 实验分组及方法同1.2.8,乳腺癌细胞与CAFs分别接种于96孔板及Transwell小室中,加入100 nmol/L E2、100nmol/LG1与2μg/mL FGF2中和抗体处理上室中CAFs细胞24 h,各组调整DMSO至一致,24 h后取出 Transwell小室,每孔加入10 μL CCK-8试剂,细胞孵箱中孵育2 h,酶标仪测定各孔的吸光度(OD值),波长选择450 nm,获得各组相对细胞数。实验重复3次。
1.3 统计学处理
数据用均数±标准差( ±s)表示,采用SPSS 17.0统计软件,多组间比较采用重复测量方差分析,两两比较采用q检验,P<0.05为差异有统计学意义。
±s)表示,采用SPSS 17.0统计软件,多组间比较采用重复测量方差分析,两两比较采用q检验,P<0.05为差异有统计学意义。
2 结 果
2.1 不同类型乳腺癌细胞均可诱导CAFs中GPER发生细胞浆转位
细胞免疫荧光实验表明:CAFs细胞单独培养条件下,GPER主要表达于细胞核,而与ER+乳腺癌MCF-7及ER-乳腺癌MDA-MB-468细胞共培养后,CAFs中GPER的表达定位由细胞核转位入细胞浆中;GPER NES序列突变后,上述两种类型的乳腺癌细胞均不能使CAFs(M)中GPER发生核浆转位,与前期结果一致[13](图1)。
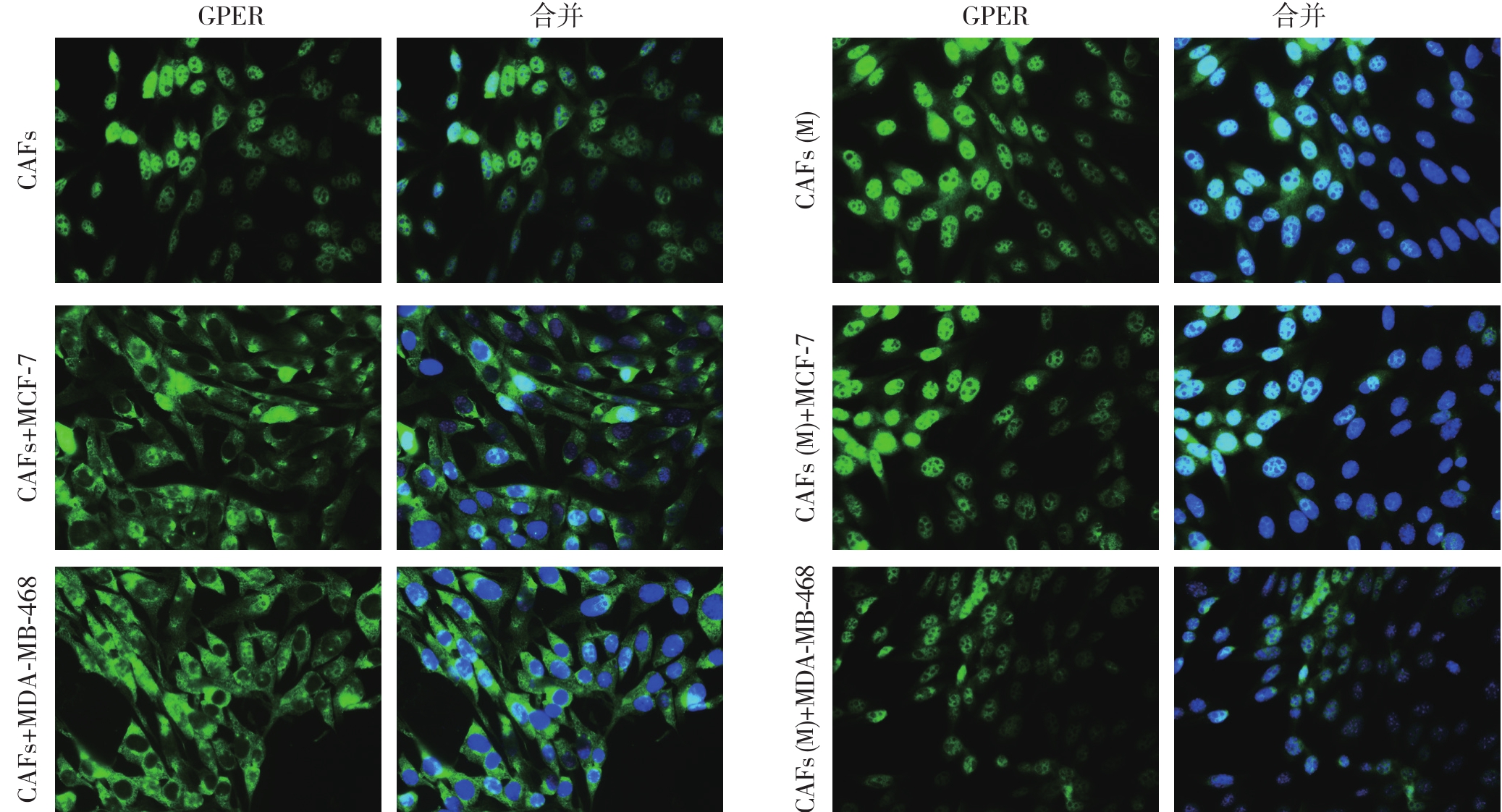
图1 免疫荧光法检CAFs细胞中GPER的表达定位(绿色荧光代表目的蛋白GPER,蓝色荧光代表细胞核;×400)
Figure 1 Determination of GPER expression localization in CAFs by immuno fl uorescence(green fl uorescence showing GPER,and blue fl uorescence showing the cell nucleus;×400)
2.2 共培养条件下,E2与G1上调CAFs细胞中FGF2的表达及分泌
实时荧光定量PCR结果显示,共培养条件下,分别用E2和G1处理CAFs细胞12 h后,均可上调细胞内FGF2 mRNA表达水平,明显高于对照组(均P<0.05)。ELISA结果进一步显示,药物处理24 h后,E2及G1明显促进共培养模型CAFs细胞上清液中FGF2的分泌量(均P<0.05)。而单独培养条件下,则无上述现象(图2)。
突变共培养模型CAFs中GPER NES序列后,E2与G1的上述作用被取消,与对照组比较无统计学差异(均P>0.05)(图3)。
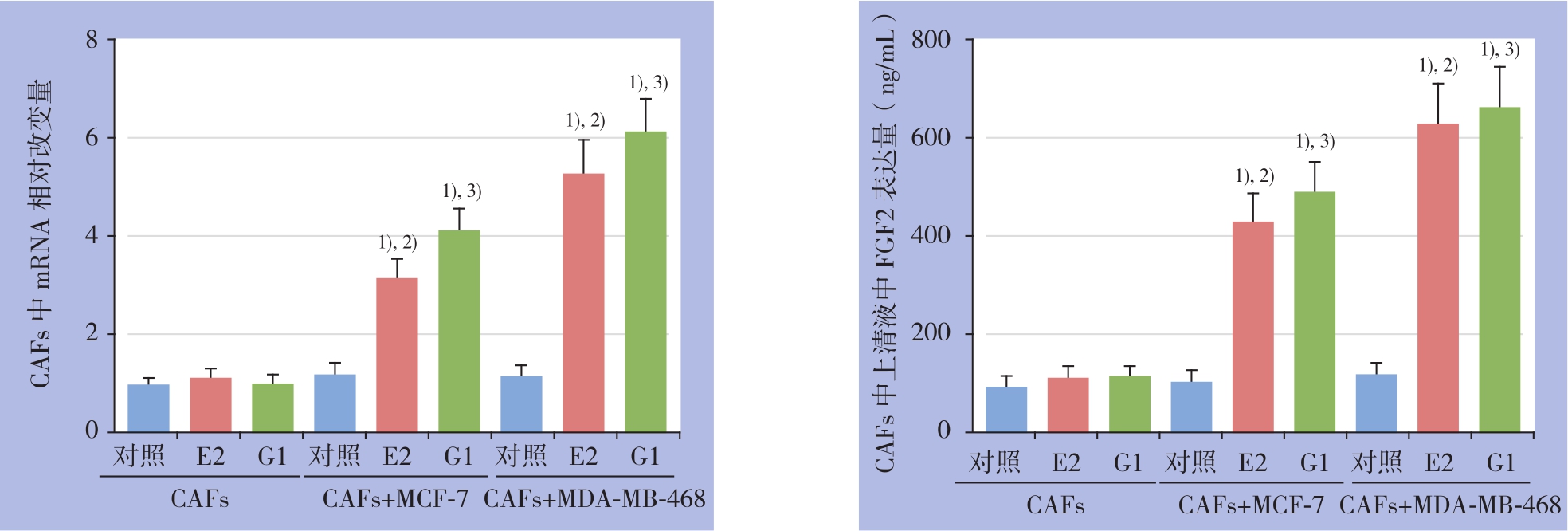
图2 荧光定量PCR及ELISA检测CAFs中FGF2 mRNA表达及上清液中FGF2含量 1)与空白对照组比较,P<0.05;2)与CAFs+E2组比较,P<0.05;3)与CAFs+G1组比较,P<0.05
Figure 2 Detection of FGF2 mRNA expression in CAFs and FGF2 level in culture supernatant by real-time qPCR and ELISA 1)P<0.05vs.blank control;2)P<0.05vs.CAFs alone+E2;3)P<0.05vs.CAFs alone+G1
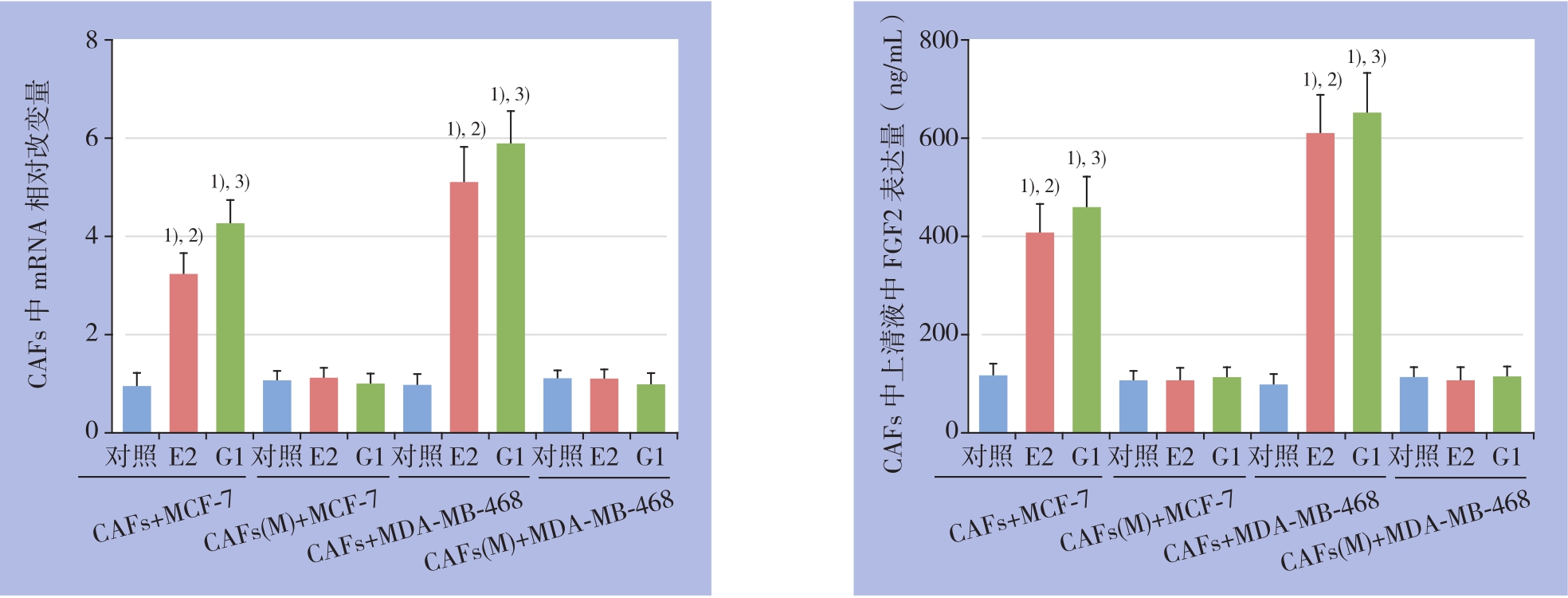
图3 突变GPER NES序列对CAFs中FGF2 mRNA表达及上清液中FGF2含量的影响 1)与对照组比较,P<0.05;2)与CAFs(M)+E2 组比较,P<0.05;3)与CAFs(M)+G1 组比较,P<0.05
Figure 3 In fl uences of GPER NES sequence mutation on FGF2 mRNA expression in CAFs and FGF2 level in culture supernatant 1)P<0.05vs.blank control;2)P<0.05vs.CAFs(M)+E2;3)P<0.05vs.CAFs(M)+G1
2.3 CAFs中细胞浆GPER介导的FGF2旁分泌促进乳腺癌细胞周期进展
细胞周期结果显示,分别用E2和G1处理共培养模型中CAFs细胞24 h后,两种药物均可降低乳腺癌MCF-7与MDA-MB-468细胞周期中静止期(G0/G1期)细胞的比例,促进细胞周期向增殖期(S期与G2/M期)进展。其中E2及G1处理后肿瘤细胞处于S期的DNA改变量最为明显(P<0.05),其促细胞周期进展的效应被FGF2的特异性中和抗体所阻断(图4)。
2.4 CAFs中细胞浆GPER介导的FGF2旁分泌增强乳腺癌细胞增殖能力
CCK-8法检测结果进一步显示,E2及G1处理CAFs细胞24 h后,可明显促进共培养模型中乳腺癌细胞增殖,且其促增殖效应被FGF2中和抗体所拮抗(图5)。
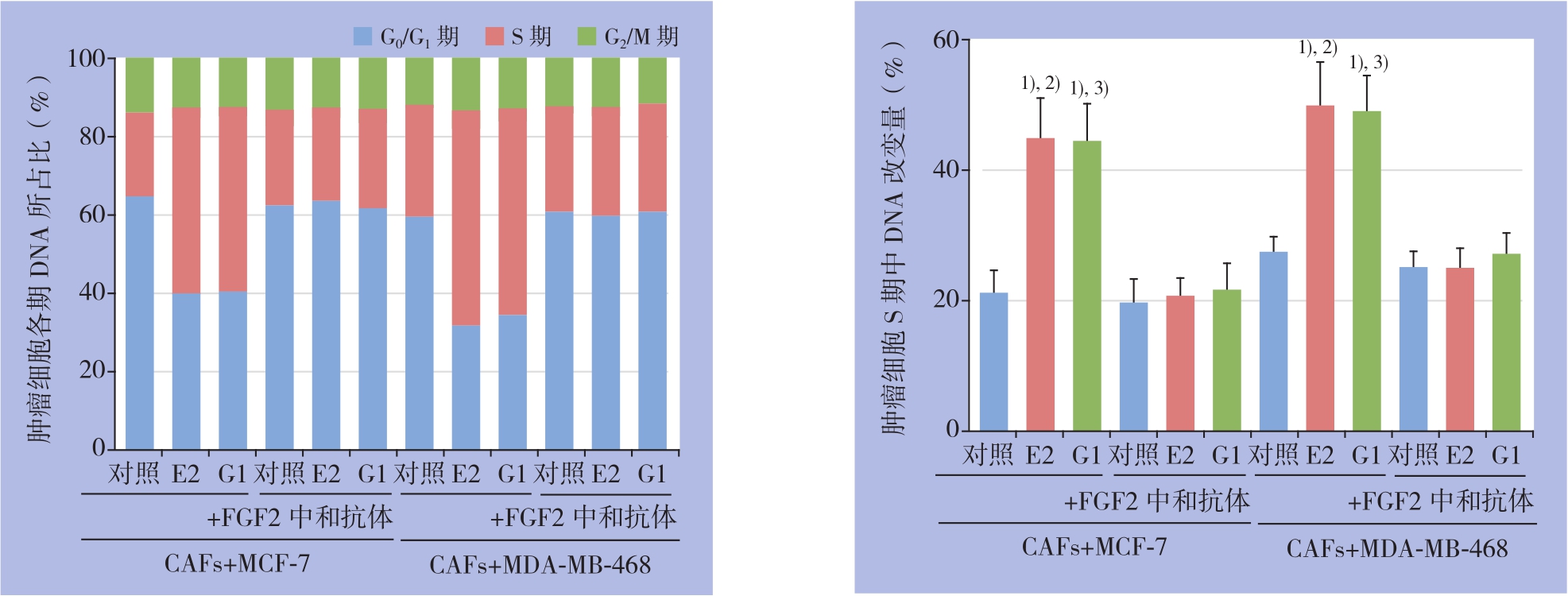
图4 流式细胞术检测共培养条件下乳腺癌细胞的细胞周期 1)与对照组比较,P<0.05;2)与E2+FGF2中和抗体组比较,P<0.05;3)与G1+FGF2中和抗体组比较,P<0.05
Figure 4 Detection of cell cycle of the breast cancer cells under co-culture condition by flow cytometry 1)P<0.05vs.control;2)P<0.05vs.E2+FGF2 neutralizing antibody;3)P<0.05vs.G1+FGF2 neutralizing antibody
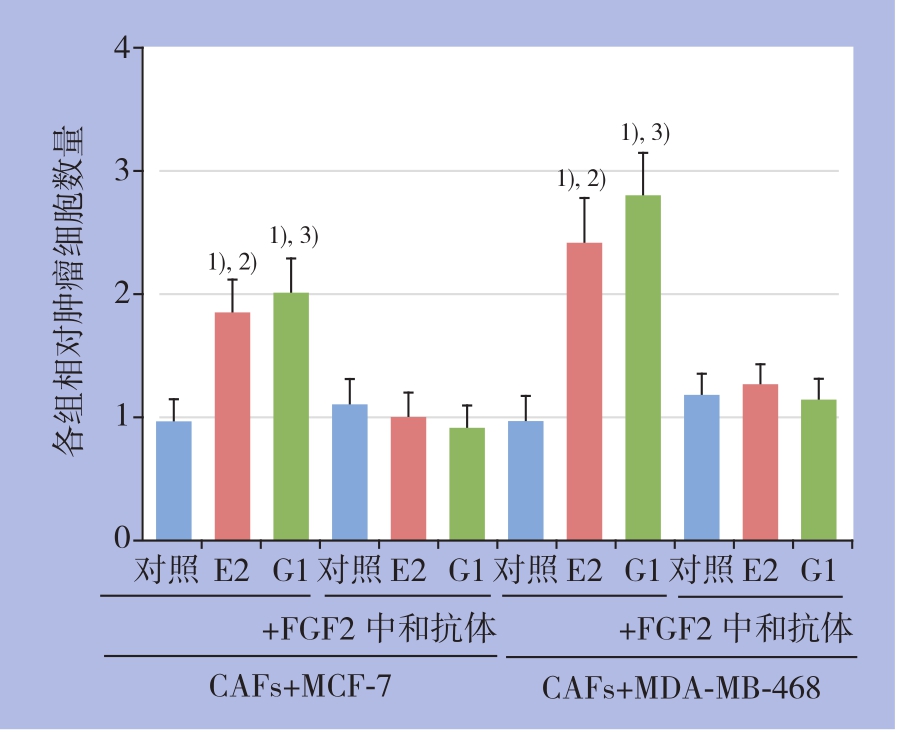
图5 CCK-8法检测共培养条件下乳腺癌细胞增殖情况1)与对照组比较,P<0.05;2)与E2+FGF2中和抗体组比较,P<0.05;3)与G1+FGF2中和抗体组比较,P<0.05
Figure 5 Determination of breast cancer cells proliferation under co-culture condition by CCK-81)P<0.05 vs.control;2)P<0.05vs.E2+FGF2 neutralizing antibody;3)P<0.05vs.G1+FGF2 neutralizing antibody
3 讨 论
肿瘤的发生发展不仅仅被认为是肿瘤实质细胞本身的单独作用,其微环境成分也同时作为肿瘤的核心特征被广泛接受[18-19]。研究团队前期体外实验数据显示CAFs单独培养条件下GPER主要表达于细胞核中,而这与免疫组化实验所观察到GPER主要表达于肿瘤间质成纤维细胞(tumor stromal fibroblasts,TSFs)中细胞浆的结果不相符合[13]。事实上,生理条件下CAFs可能受到肿瘤组织中多种成分的影响,而肿瘤实质细胞与CAFs是乳腺肿瘤中最主要的两大成分,因此提示在体内环境乳腺癌细胞可能影响CAFs中GPER的细胞内定位。仅仅单独研究CAFs中GPER的生物学功能可能是不妥的,乳腺癌细胞与CAFs之间的“交叉对话”须考虑在内,这更加符合实体肿瘤真实的“内部环境”。本研究在不同类型的乳腺癌(ER+型MCF-7与ER-型MDA-MB-468)细胞中均证实了GPER的核浆转位现象,提示CAFs中GPER的核浆转位可能是乳腺肿瘤的普遍特点之一,其在肿瘤进展及药物耐受中或发挥关键性作用,可能是潜在的治疗靶点。
近年来,GPER所介导的肿瘤旁分泌效应越来越被诸多研究者所关注。郭林英等[20]报道CAFs中GPER可介导细胞因子HMGB1外分泌促进ER+乳腺癌细胞自噬。此外,在ER-乳腺癌细胞中,雌激素还可通过活化GPER自分泌炎症因子IL-6进而促进肿瘤细胞快速生长[21]。同样,笔者在前期研究中也证实GPER创造乳酸微环境引起乳腺癌细胞能量代谢重塑从而导致肿瘤细胞的临床多药耐受[13]。本研究进一步明确了乳腺癌微环境GPER与成纤维细胞因子家族成员FGF2的潜在调控关系:共培养条件下雌激素活化CAFs中细胞浆GPER促进FGF2的表达及旁分泌,突变GPER的NES序列(GPER位于CAFs细胞核)后可阻断上述效应。同时,CAFs单独培养条件下则未观察到上述现象,更进一步提示共培养下微环境GPER细胞浆定位的独特生物学作用。然而,CAFs中雌激素/GPER/FGF2信号轴活化的具体机制尚不明确,有待后续实验的进一步研究。
现有研究表明,FGF2的过量表达及下游信号的过度活化参与肿瘤恶性临床进展[22-23]。在慢性髓系白血病(chronic myeloid leukemia,CML)中,FGF2表达异常升高将导致CML细胞对靶向药物伊马替尼治疗不敏感[24]。Terai等[25]则证实非小细胞肺癌中FGF2及其受体FGFR1活性增强将引起肿瘤细胞快速增殖及转移,且对靶向药物吉非替尼出现耐药。同样,本研究也得到类似结果:微环境GPER所介导的FGF2旁分泌可显著促进乳腺癌细胞周期进展及肿瘤快速生长,应用FGF2的特异性中和抗体可明显逆转以上效应,提示靶向阻断CAFs中雌激素/GPER/FGF2信号通路可能是对抗肿瘤临床进展的新型治疗模式,但这还需要更多的体外及体内实验来进一步验证与支持。
[1]Houthuijzen JM,Jonkers J.Cancer-associated fibroblasts as key regulators of the breast cancer tumor microenvironment[J].Cancer Metastasis Rev,2018,37(4):577-597.doi:10.1007/s10555-018-9768-3.
[2]孙振华,赵志泓,蒋华平,等.肿瘤微环境相关因子IL-6、IL-10、CXCR7在甲状腺乳头状癌中的表达及临床意义[J].中国普通外科杂志,2017,26(5):578-582.doi:10.3978/j.issn.1005-6947.2017.05.008.Sun ZH,Zhao ZH,Jiang HP,et al.Expressions of tumor microenvironment-associated factors IL-6,IL-10 and chemokine receptor7 in papillary thyroid carcinoma and their clinical significance[J].Chinese Journal of General Surgery[J].Chinese Journal of General Surgery,2017,26(5):578-582.doi:10.3978/j.issn.1005-6947.2017.05.008.
[3]Plava J,Cihova M,Burikova M,et al.Recent advances in understanding tumor stroma-mediated chemoresistance in breast cancer[J].Mol Cancer,2019,18(1):67.doi:10.1186/s12943-019-0960-z.
[4]李开富,张晓耀.肿瘤相关成纤维细胞在乳腺癌内分泌治疗中作用的研究进展[J].中国普通外科杂志,2015,24(5):733-738.doi:10.3978/j.issn.1005-6947.2015.05.023.Li KF,Zhang XY.Research progress in the role of carcinomaassociated fibroblasts in endocrine therapy for breast cancer[J].Chinese Journal of General Surgery,2015,24(5):733-738.doi:10.3978/j.issn.1005-6947.2015.05.023.
[5]LappanOR,Maggiolini M.GPER is involved in the functional liaison between breast tumor cells and cancer-associated fibroblasts(CAFs)[J].J Steroid Biochem Mol Biol,2018,176:49-56.doi:10.1016/j.jsbmb.2017.02.019.
[6]Yu T,Liu M,Luo H,et al.GPER mediates enhanced cell viability and motility via non-genomic signaling induced by 17β-estradiol in triple-negative breast cancer cells[J].J Steroid Biochem Mol Biol,2014,143:392-403.doi:10.1016/j.jsbmb.2014.05.003.
[7]Yin H,Zhu Q,Liu M,et al.GPER promotes tamoxifen-resistance in ER+ breast cancer cells by reduced Bim proteins through MAPK/Erk-TRIM2 signaling axis[J].Int J Oncol,2017,51(4):1191-1198.doi:10.3892/ijo.2017.4117.
[8]Li Y,Jia Y,Bian Y,et al.Autocrine motility factor promotes endometrial cancer progression by targeting GPER-1[J].Cell Commun Signal,2019,17(1):22.doi:10.1186/s12964-019-0336-4.
[9]Hernández Silva CD,Riera-Leal A,Ortiz-Lazareno PC,et al.GPER Overexpression in Cervical Cancer Versus Premalignant Lesions:Its Activation Induces Different forms of Cell Death[J].Anticancer Agents Med Chem,2019.doi:10.2174/1871520619666190206171509.[Epub ahead of print]
[10]Cortes E,Sarper M,Robinson B,et al.GPER is a mechanoregulator of pancreatic stellate cells and the tumor microenvironment[J].EMBORep,2019,20(1).pii:e46556.doi:10.15252/embr.201846556.
[11]Luo H,Liu M,Luo S,et al.Dynamic monitoring of GPER-mediated estrogenic effects in breast cancer associated fibroblasts:An alternative role of estrogen in mammary carcinoma development[J].Steroids,2016,112:1-11.doi:10.1016/j.steroids.2016.03.013.
[12]Luo H,Yang G,Yu T,et al.GPER-mediated proliferation and estradiol production in breast cancer-associated fibroblasts[J].Endocr Relat Cancer,2014,21(2):355-369.doi:10.1530/ERC-13-0237.
[13]Yu T,Yang G,Hou Y,et al.Cytoplasmic GPER translocation in cancer-associated fibroblasts mediates cAMP/PKA/CREB/glycolytic axis to confer tumor cells with multidrug resistance[J].Oncogene,2017,36(15):2131-2145.doi:10.1038/onc.2016.370.
[14]Akl MR,Nagpal P,Ayoub N M,et al.Molecular and clinical significance of fibroblast growth factor 2(FGF2 /bFGF)in malignancies of solid and hematological cancers for personalized therapies[J].Oncotarget,2016,7(28):44735-44762.doi:10.18632/oncotarget.8203.
[15]Javidi-SharifiN,Martinez J,English I,et al.FGF2-FGFR1 signaling regulates release of Leukemia-Protective exosomes from bone marrow stromal cells[J].Elife,2019,pii:e40033.doi:10.7554/eLife.40033.
[16]Awaji M,Futakuchi M,Heavican T,et al.Cancer-Associated Fibroblasts Enhance Survival and Progression of the Aggressive Pancreatic Tumor Via FGF-2 and CXCL8[J].Cancer Microenviron,2019.doi:10.1007/s12307-019-00223-3.[Epub ahead of print]
[17]Seo JH,Yu JH,Suh H,et al.Fibroblast growth factor-2 induced by enriched environment enhances angiogenesis and motor function in chronic hypoxic-ischemic brain injury[J].PLoS One,2013,8(9):e74405.doi:10.1371/journal.pone.0074405.
[18]Qiao A,Gu F,Guo X,et al.Breast cancer-associated fibroblasts:their roles in tumor initiation,progression and clinical applications[J].Front Med,2016,10(1):33-40.doi:10.1007/s11684-016-0431-5.
[19]邢荣春,秦周萍.胰腺导管腺癌肿瘤微环境的研究进展[J].中国普通外科杂志,2017,26(9):1202-1206.doi:10.3978/j.issn.1005-6947.2017.09.019.Xing RC,Qin ZP.Research progress of tumor microenvironment in pancreatic ductal adenocarcinoma[J].Chinese Journal of General Surgery,2017,26(9):1202-1206.doi:10.3978/j.issn.1005-6947.2017.09.019.
[20]郭林英,余腾骅,张光君,等.肿瘤相关成纤维细胞中GPER介导HMGB1外分泌促进乳腺癌MCF-7细胞自噬及增殖[J].肿瘤,2017,37(5):448-456.Guo LY,Yu TH,Zhang GJ,et al.GPER-mediated HMGB1 exocrine in cancer-associated hbroblasts promotes autophagy and proliferation of breast cancer MCF-7 cells[J].Tumor,2017,37(5):448-456.
[21]王健,徐杰,安雪青,等.雌激素促进ER阴性乳腺癌细胞中IL-6的表达[J].南京医科大学学报:自然科学版,2015,35(3):309-314.doi:10.7655/NYDXBNS20150303.Wang J,Xu J,An XQ,et al.Estrogen enhances expression of interleukin-6 in ER-negative breast cancer cells[J].Journal of Nanjing Medicial University,2015,35(3):309-314.doi:10.7655/NYDXBNS20150303.
[22]Miura K,Oba T,Hamanaka K,et al.FGF2-FGFR1 pathway activation together with thymidylate synthase upregulation is induced in pemetrexed-resistant lung cancer cells[J].Oncotarget,2019,10(11):1171-1192.doi:10.18632/oncotarget.26622.
[23]李斌德,颜禄斌,沈阳,等.转化生长因子β1与碱性成纤维生长因子与儿童先天性胆管扩张症的关系[J].中国普通外科杂志,2015,24(2):283-287.doi:10.3978/j.issn.1005-6947.2015.02.025.Li BD,Yan LB,Shen Y,et al.Relationship of transforming growth factor B1 and basic fibroblast growth factor with congenital choledochal cyst in children[J].Chinese Journal of General Surgery,2015,24(2):283-287.doi:10.3978/j.issn.1005-6947.2015.02.025.
[24]Traer E,Javidi-SharifiN,Agarwal A,et al.Ponatinib overcomes FGF2-mediated resistance in CML patients without kinase domain mutations[J].Blood,2014,123(10):1516-1524.doi:10.1182/blood-2013-07-518381.
[25]Terai H,Soejima K,Yasuda H,et al.Activation of the FGF2-FGFR1 autocrine pathway:a novel mechanism of acquired resistance to ge fitinib in NSCLC[J].Mol Cancer Res,2013,11(7):759-767.doi:10.1158/1541-7786.MCR-12-0652.
