肝门部胆管癌是指位于胆囊管开口以上的肝总管、左右肝管汇合部和左右肝管的胆管黏膜上皮癌,是肝外胆管癌最常见的一类,约占40%~60%[1-3]。由于肝门区特殊的解剖部位及肿瘤纵向侵犯、横向浸润的生物学特性,肝门部胆管癌患者根治切除率低,预后不佳,5年总生存率仅为10%左右[4]。对于不可切除的肝门部胆管癌,胆管梗阻常引起厌食、皮肤瘙痒、胆管炎等症状[5],有效解除梗阻、去除黄疸是主要的治疗方案,经皮肝胆管穿刺置管引流术(PTCD)操作简单,可有效引流胆汁,减轻黄疸,是目前不可切除肝门部胆管癌常用的治疗方式[6],然而其并未直接作用于肿瘤细胞,且存在管道移位、脱落、阻塞等并发症,常对患者产生不适,增加生活负担[7]。
纳米刀消融术(nanoknife ablation)通过高压电脉冲,在肿瘤细胞膜上形成不可逆的纳米级通道,增加细胞膜的通透性,细胞破坏后失去内稳态,最终导致肿瘤细胞凋亡,并实现胆道再通[8]。目前临床上已应用于胰腺癌、肝癌等的治疗[9],而对不可切除肝门部胆管癌报道相对较少。国外有研究显示,纳米刀消融术后可实现胆管再通[10],本文通过分析15例纳米刀消融治疗不可切除肝门部胆管癌患者,初步总结消融术后不同时间胆管再通率,观察患者生活质量改善情况、近期生存期等,以供参考。
1 资料与方法
1.1 一般资料
将郑州大学第五附属医院2016年7月—2017年7月收治的35例不可切除肝门部胆管癌患者分为观察组(15例)和对照组(20例),两组患者年龄、肿瘤分型、性别等一般资料比较,差异无统计学意义(均P>0.05),具有可比性(表1)。两组术前均行增强CT或MRI等检查,明确梗阻部位及肝内胆管扩张程度后行PTC D 治疗,观察组行PTCD治疗后1~2周,待胆红素下降、肝功能改善后行开腹纳米刀消融术;对照组单纯行PTC D治疗。本研究通过郑州大学第五附属医院伦理委员会审查同意,所有患者以及家属对本次研究均知情同意,并且自愿参与本次研究。纳入标准: ⑴ 术前影像学检查、血清肿瘤标记物、肝功能、及临床症状等资料,结合穿刺病理检查确诊为肝门部胆管癌;⑵ 依据Bismuth-Corlette分型[11],均为III~IV型肝门部胆管癌患者,肿瘤侵犯大血管,无手术切除条件且无远处转移;⑶ 重要脏器(心、肝、肾、肺)功能无明显异常,能耐受开腹手术;⑷ 患者依从性好,能定期复查,且接受长期随访。排除标准:⑴ 患者一般条件差,不能耐受全身麻醉及手术;⑵ 依从性差,不能定期复查和接受随访患者。
1.2 手术方法
观察组接受纳米刀消融与PTCD 联合治疗,在CT引导下选择穿刺靶点后,实施局部麻醉,采用穿刺针(18 G)实施穿刺,达到靶点后,拔出针芯,将导丝置于肝门部,沿导丝植入猪尾巴,将导丝拔出,使猪尾巴管固定在皮肤上。待患者黄疸缓解,肝管改善后行纳米刀消融术,常规开腹,充分游离第一肝门周围组织,暴露肿瘤;术中超声精确测量肿瘤大小、形态,进一步确定肿瘤侵犯部位、与周围重要血管关系。将测得数据输入纳米刀消融系统,制订布针计划,超声监测下,设计布针位置、进针方向。选用15 cm长的单极消融电极针1 根作为主针,辅针2 ~3 根精准布针,暴露消融电极深度为1.5~2.5 cm,相邻电极针间距为1.5~2.5 cm,布针后再次超声检测是否达到肿瘤底部及边缘,采用直流(25 A)高压 (2500~3000 V)电脉冲依次消融,每组脉冲消融间隔70 µs,共计90~130次,根据消融过程后电流上升梯度,决定是否追加脉冲次数,若肿瘤较大,可以分次、分部位布针消融。术后监测生命体征变化情况,禁食水,积极给予保肝、抗炎、静脉营养支持等治疗。
对照组仅接受经皮肝胆管穿刺置管引流治疗,操作方式与观察组一致。
表1 两组患者一般资料比较
Table1 Comparison of general data between two groups of patients
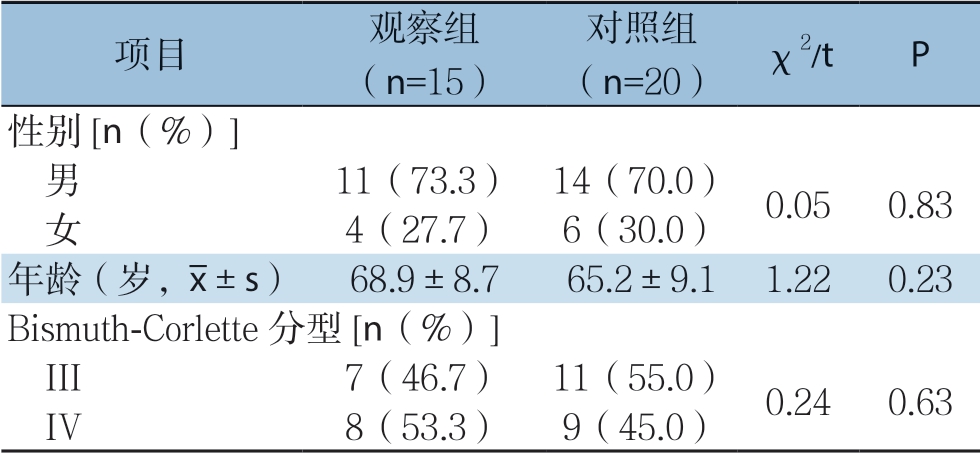
项目 观察组(n=15)对照组(n=20) χ2/t P性别[n(%)] 男 11(73.3) 14(70.0) 0.050.83 女 4(27.7) 6(30.0)年龄(岁,images/BZ_72_1484_1247_1506_1298.png±s) 68.9±8.765.2±9.11.220.23 Bismuth-Corlette 分型[n(%)] III 7(46.7) 11(55.0) 0.240.63 IV 8(53.3) 9(45.0)
1.3 观察指标及随访
⑴ 监测两组患者术前及术后1、3 个月的肝功能:总胆红素(TBIL)、丙氨酸氨基转移酶(ALT)、天门冬氨酸氨基转移酶(AST)变化情况;⑵ 采用肿瘤患者生活质量量表(EORTC QLQ-C30 V3.0 中文版)评分[12]分析所有患者术前及术后1、3个月生活质量评分情况,将原始数据通过加权平均和线性转换使其得分在1~100之间;⑶ 统计两组患者生存时间,记录两组术后并发症发生情况;⑷ 所有患者均随访2年,嘱患者术后1、3个月规律来院复查血常规、肝功及增强CT等检查,明确患者肝功能、胆道是否通畅及生化质量情况,必要时经PTCD管行造影检查,随访截至时间为2019年7月或患者死亡。
1.4 统计学处理
采用SPSS 21.0软件对数据进行统计分析。计量资料以均数±标准差(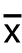 ±s)表示,组间比较采用独立样本t检验,组内比较采用配对t检验;计数资料以率(%)表示,组间比较采用χ2检验;生存分析采用Kaplan-Meier生存曲线分析法,组间比较采用Log-rank检验,以P<0.05为差异具有统计学意义。
±s)表示,组间比较采用独立样本t检验,组内比较采用配对t检验;计数资料以率(%)表示,组间比较采用χ2检验;生存分析采用Kaplan-Meier生存曲线分析法,组间比较采用Log-rank检验,以P<0.05为差异具有统计学意义。
2 结 果
2.1 生化指标
观察组与对照组手术成功率均为100%,治疗前两组患者ALT、AST、总胆红素水平比较,差异均无统计学意义(均P>0.05);观察组术后1、3个月ALT、AST、总胆红素较术前明显下降,差异有统计学意义(均P <0.05);对照组术后 1个月ALT、AST、总胆红素较术前下降,差异有统计学意义(P<0.05),术后3个月与术前相比差异无统计学意义(P>0.05);观察组术后1、3个月上诉指标均优于对照组,差异有统计学意义(P<0.05)(表2)。
表2 两组患者治疗前后转氨酶与TBIL 水平比较(x-±s)
Table2 Comparison of transaminase and TBIL levels between the two groups before and after treatment (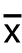 ±s)
±s)
注:1)与本组术前比较,P<0.05
Note:1) P<0.05 vs.the same group before surgery
指标 观察组(n=15)对照组(n=20) t P ALT(U/L) 术前1 d 91.2±57.696.1±55.10.240.81 术后1 个月 45.3±18.11) 65.8±37.11) 2.710.01 术后3 个月 27.2±8.11) 92.3±61.34.570.00 AST(U/L) 术前1 d 94.8±37.790.2±40.10.340.73 术后1 个月 47.1±14.31) 64.1±32.81) 2.060.04 术后3 个月 29.9±8.31) 87.8±34.77.200.00 TBIL(μmol/L) 术前1 d 192.4±76.1188.9±63.50.150.89 术后1 个月 69.8±45.71) 126.5±85.71) 2.520.02 术后3 个月 27.4±38.61) 183.4±71.38.230.00
2.2 生存质量
两组患者术前生存质量相比差异无统计学意义(P >0.05),观察组术后1、3 个月生存质量较其术前明显好转,差异具有统计学意义(P<0.05);对照组术后1个月生存质量较术前好转,差异有统计学意义(P<0.05),术后3个月余术前相比差异无统计学意义(P>0.05);观察组术后1、3个月生存质量均优于对照组,差异有统计学意义(均P<0.05)(表3)。
表3 两组患者手术前后生活质量变化(x-±s)
Table3 Comparison of the patients’quality of life between the two groups before and after operation ( 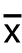 ±s)
±s)
注:1)与本组术前比较,P<0.05
Note:1) P<0.05 vs.the same group before surgery
组别 生活质量评分术前1 d 术后1 个月 术后3 个月观察组(n=15) 42.1±5.766.3±14.91) 73.6±9.61)对照组(n=20) 45.6±.9.254.7±12.41) 47.9±13.6 t 1.342.066.22 P 0.180.040.00
2.3 生存时间、胆管通畅时间及术后因PTCD 管梗阻、感染再次住院情况
随访至2019年7月,观察组中位生存时间为15 个月,对照组为5 个月,观察组明显优于对照组(P <0.05)。观察组1、2年生存率为80.0%、6.7%,对照组为15.0%、0,观察组术后1年累积生存率明显高于对照组(P<0.05),两组术后2年累积生存率相比差异无统计学意义(P>0.05)(图1)。观察组术后经PTCD管造影显示,所有患者胆道均可再通,胆管通畅时间为(185.1±95.8)d,术后1、3个月PTCD管拔出率为82.6%、98.0%,拔出前无患者因PTCD管梗阻、感染再次住院。
2.4 术后不良反应及并发症
观察组术后部分患者出现腹痛、发热等不良反应,给予对症治疗后症状缓解,根据Clavien-Dindo分级[13]均在1~2级,术后发生胆道感染2例,上消化道出血1 例,经积极治疗后均缓解,术后 3个月内无胆瘘、腹腔感染、腹腔内出血等并发症发生;对照组胆管梗阻进行性加重,术后3个月内因PTCD管梗阻、感染再次住院率为50%。
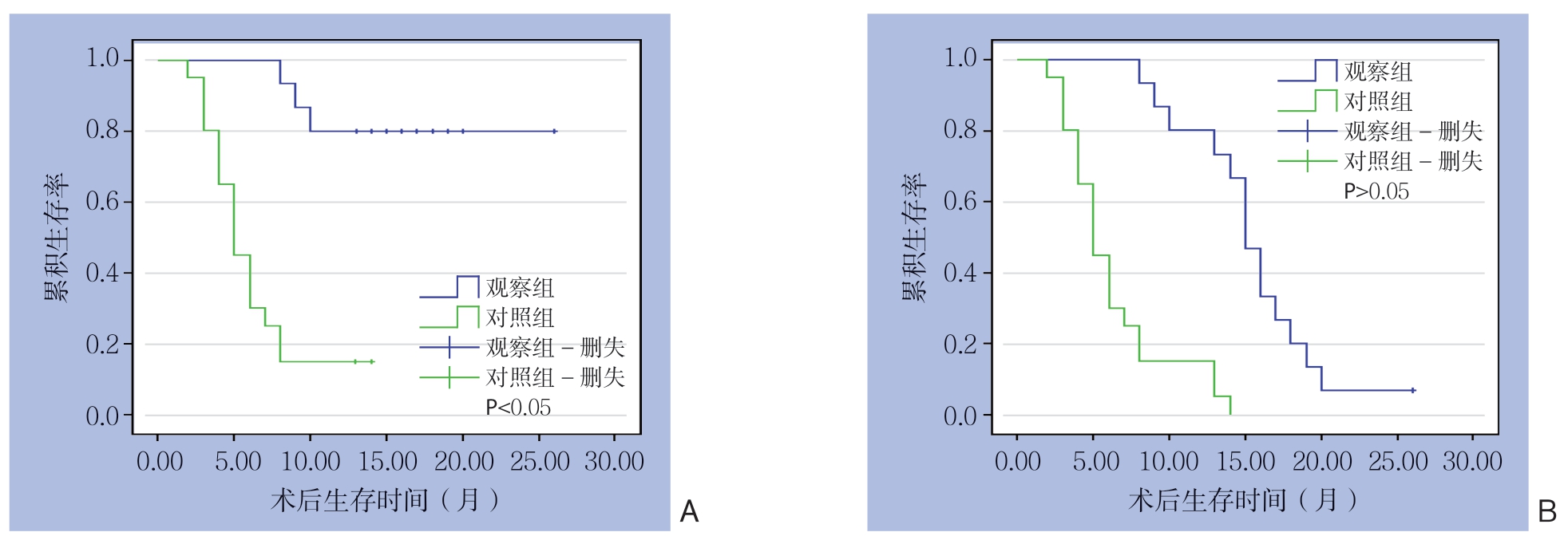
图1 两组患者的生存曲线
Figure1 Survival curves of the two groups of patients
A:1年生存率;B:2年生存率
A:1-year survival rates; B:2-year survival rates
3 讨 论
肝门部胆管癌早期症状不典型,恶行程度高,早期即会出现尾状叶侵犯、淋巴结转移、神经浸润、侵犯肝动脉及门静脉等特点,其根治率低,仅为20%~60%,预后极差。胆道恶性肿瘤引起高胆红素血症,造成神经、泌尿、循环等系统损害,严重影响患者生存质量。目前对不可切除的肝门部胆管癌主要治疗方法包括姑息性手术切除、单纯引流、放化疗、光动力疗法、肝移植等。肝门部胆管癌对放化疗不敏感[14],光动力学疗法具有肿瘤杀伤半径小及皮肤光毒性等缺点[15]。因此,不可切除肝门部胆管癌总体治疗效果不理想。
对于Bismuth III~IV型不可切除肝门部胆管癌,PTCD与内镜引流相比,成功率更高,国外多个研究认为PTCD是此类患者胆道减压的首选方法[16-17]。本研究中6例患者因内镜下放置胆管支架失败后改行PTCD治疗,均获得成功,与国外研究相似。但随着肿瘤的进展,引流管会被肿瘤侵犯或压迫,导致黄疸症状复发,患者因引流管梗阻或感染需再次住院,加重了患者生存负担。本研究对照组患者术后1个月肝功能及生存质量较术前好转,显示PTCD引流可在一定程度上改善病情,然而术后 3个月结果显示患者因PTCD管梗阻或感染再次住院率达到50%,与国外研究相似[10,18],且肝功能指标回升,证明单纯引流,缓解胆管梗阻效果不佳,应同时治疗肿瘤组织。
纳米刀消融是一种新型的非热能肿瘤消融技术,它利用高压脉冲电场,破坏肿瘤细胞膜表面的稳定性,在细胞膜上产生不可逆的纳米级通道,导致细胞内渗透压上升,最终通过凋亡导致肿瘤细胞死亡,基本不影响血管弹性纤维、细胞基质,可以有效的保护周围的血管、胆管等重要组织[8,19-21]。对侵犯血管、胆管等周围重要组织而无法切除肝门部胆管癌患者尤为适用。此外纳米刀消融与传统消融(RFA、HIFU)相比,不存在热沉降效应,可以彻底消融肿瘤区,显著提高消融效果,因此对侵犯周围血管等重要组织结构的无法根治切除肝门部胆管癌有特殊优势[22-25]。研究[26]显示,纳米刀消融通过对肿瘤细胞的灭活,可实现胆道再通,具有改善肝管能、提高生活质量、延长生存率及术后并发症发生率低的优势。Martin等[10]报告了26例肝门部胆管癌患者行IRE治疗,术前患者均行PTCD减黄治疗,结果显示纳米刀消融术后通过PTCD管造影显示所有患者胆道均可再通,患者均可拔出PTCD管,患者PTCD管置入至拔出中位时间为427 d,IRE术后至PTCD管拔出中位时间为122 d,胆道通畅中位时间为305 d,单纯PTCD治疗患者术后胆管梗阻、胆管炎等并发症发生率为59%。与观察组结果相似,观察组术后经PTCD 造影显示所有患者胆管均可再通,术后胆管通畅时间为(185.1±95.8)d,术后1、3个月PTCD管拔出率为82.6%、98%,拔出前无患者因PTCD管梗阻、感染再次住院,且术后1、3个月 肝功能指标显著优于对照组;对照组胆管梗阻进行性加重,术后3个月内因PTCD管梗阻、感染再次住院率为50%,进一步显示纳米刀消融通过杀灭肿瘤细胞实现胆管再通,改善肝功能从而为患者外引流管拔出提供机会,提高了患者生存质量,减轻其生活负担。观察组患者中位生存期为15个月, 显著优于对照组,表明纳米刀消融对肿瘤细胞具有良好的灭活作用,显著延长患者生存期。
观察组术后发生胆道感染2例,与Martin等[10]研究相似,考虑患者长时间胆道梗阻致胆汁粘稠,加之纳米刀消融手术时多次布针可能刺激胆管周围组织水肿,压迫胆管,以上均是胆道感染有利因素。术后上消化道出血1例,现无明确证据表明在肝门部胆管癌中术后上消化道出血与纳米刀消融有关,但在胰腺纳米刀消融中出血与消融有相关性[27-30],考虑纳米刀消融过程中电极针之间产生热量向周围辐射,损伤血管内皮细胞所致,但本例患者黄疸时间长,凝血功能差,也不完全排除应激性溃疡可能,因此在消融中要保持针距0.5 cm以上,若消融次数多,应更换电极针的正负极,对凝血功能差的病人,术后积极给予抑酸、护胃等治疗。
综上所述,纳米刀消融可有效的灭活肿瘤细胞,改善肝功能,实现胆道再通,提高患者生存质量,给不可切除的肝门部胆管癌患者带来了福音。国内外临床研究表明,作为治疗肝门部胆管癌的一种新方法,纳米刀消融安全、有效。然而,目前对于纳米刀消融术后拔除PTCD时机,消融过程中的最佳参数选择,使用电极针的数量等方面尚无明确标准,有待进一步研究。
[1] Dumitrascu T,Chirita D,Ionescu M,et al.Resection for hilar cholangiocarcinoma:analysis of prognostic factors and the impact of systemic inflammation on long-term outcome[J].J Gastrointest Surg,2013,17(5):913–924.doi:10.1007/s11605–013–2144–2.
[2] Young AL,Igami T,Senda Y,et al.Evolution of the surgical management of perihilar cholangiocarcinoma in a Western centre demonstrates improved survival with endoscopic biliary drainage and reduced use of blood transfusion[J].HPB (Oxford),2011,13(7):483–493.doi:10.1111/j.1477–2574.2011.00328.x.
[3] Razumilava N,Gores GJ.Cholangiocarcinoma[J].Lancet,2014,383(9935):2168–2179.doi:10.1016/S0140–6736(13)61903–0.
[4] Hasegawa S,Ikai I,Fujii H,et al.Surgical resection of hilar cholangiocarcinoma:analysis of survival and postoperative complications[J].World J Surg,2007,31(6):1256–1263.doi:10.1007/s00268–007–9001-y.
[5] Mansour JC,Aloia TA,Crane CH,et al.Hilar cholangiocarcinoma:expert consensus statement[J].HPB (Oxford),2015,17(8):691–699.doi:10.1111/hpb.12450.
[6] Duan F,Cui L,Bai Y,et al.Comparison of efficacy and complications of endoscopic and percutaneous biliary drainage in malignant obstructive jaundice:a systematic review and metaanalysis[J].Cancer Imaging,2017,17(1):27.doi:10.1186/s40644–017–0129–1.
[7] Tang Z,Yang Y,Meng W,et al.Best option for preoperative biliary drainage in Klatskin tumor:A systematic review and metaanalysis[J].Medicine (Baltimore),2017,96(43):e8372.doi:10.1097/MD.0000000000008372.
[8] Vogel JA,van Veldhuisen E,Agnass P,et al.Time-Dependent Impact of Irreversible Electroporation on Pancreas,Liver,Blood Vessels and Nerves:A Systematic Review of Experimental Studies[J].PLoS One,2016,11(11):e0166987.doi:10.1371/journal.pone.0166987.
[9] 刘少朋,李晓勇,程冰冰.纳米刀消融术治疗局部晚期不可切除胰腺癌安全性及疗效评价[J].中国普通外科杂志,2016,25(9):1259–1265.doi:10.3978/j.issn.1005–6947.2016.09.006.
Liu SP,Li XY,Cheng BB.Safety and efficacy of nanoknife ablation for locally advanced unresectable pancreatic cancer[J].Chinese Journal of General Surgery,2016,25(9):1259–1265.doi:10.3978/j.issn.1005–6947.2016.09.006.
[10] Martin EK,Bhutiani N,Egger ME,et al.Safety and efficacy of irreversible electroporation in the treatment of obstructive jaundice in advanced hilar cholangiocarcinoma[J].HPB (Oxford),2018,20(11):1092–1097.doi:10.1016/j.hpb.2018.06.1806.
[11] Liska V,Treska V,Skalicky T,et al.Evaluation of Tumor Markers and Their Impact on Prognosis in Gallbladder,Bile Duct and Cholangiocellular Carcinomas - A Pilot Study[J].Anticancer Res,2017,37(4):2003–2009.doi:10.21873/anticanres.11544.
[12] 王晓燕,高彤.肠外营养联合放化疗治疗上消化道恶性肿瘤的临床观察[J].临床肿瘤学杂志,2018,23(9):840–844.doi:10.3969/j.issn.1009–0460.2018.09.014.
Wang XY,Gao T.Clinical observation of parenteral nutrition combined with radiotherapy and chemotherapy in the treatment of upper gastrointestinal malignant tumors[J].Chinese Clinical Oncology,2018,23(9):840–844.doi:10.3969/j.issn.1009–0460.2018.09.014.
[13] Dindo D,Demartines N,Clavien PA.Classification of surgical complications:a new proposal with evaluation in a cohort of 6336 patients and results of a survey[J].Ann Surg,2004,240(2):205–213.doi:10.1097/01.sla.0000133083.54934.ae.
[14] Noji T,Tsuchikawa T,Okamura K,et al.Concomitant hepatic artery resection for advanced perihilar cholangiocarcinoma:a case-control study with propensity score matching[J].J Hepatobiliary Pancreat Sci,2016,23(7):442–448.doi:10.1002/jhbp.363.
[15] 闫炫炫,李多富,李汛.光动力疗法在不可切除肝门部胆管癌中的应用[J].中国普外基础与临床杂志,2018,25(4):488–492.doi:10.7507/1007–9424.201708068.
Yan XX,Li DF,Li X.Application of photodynamic therapy in palliative treatment of unresectable hilar cholangiocarcinoma[J].Chinese Journal of Bases and Clinics in General Surgery,2018,25(4):488–492.doi:10.7507/1007–9424.201708068.
[16] Moole H,Dharmapuri S,Duvvuri A,et al.Endoscopic versus Percutaneous Biliary Drainage in Palliation of Advanced Malignant Hilar Obstruction:A Meta-Analysis and Systematic Review[J].Can J Gastroenterol Hepatol,2016,2016:4726078.doi:10.1155/2016/4726078.
[17] Boulay BR,Birg A.Malignant biliary obstruction:From palliation to treatment[J].World J Gastrointest Oncol,2016,8(6):498–508.doi:10.4251/wjgo.v8.i6.498.
[18] Nennstiel S,Weber A,Frick G,et al.Drainage-related Complications in Percutaneous Transhepatic Biliary Drainage:An Analysis Over 10 Years[J].J Clin Gastroenterol,2015,49(9):764–770.doi:10.1097/MCG.0000000000000275.
[19] Kang BC,Lee SW,Chung HH.A newly designed Y-shaped covered stent in the palliative treatment of hepatic hilar malignant obstruction:case report[J].Korean J Radiol,2013,14(1):97–101.doi:10.3348/kjr.2013.14.1.97.
[20] Rubinsky B,Onik G,Mikus P.Irreversible electroporation:a new ablation modality--clinical implications[J].Technol Cancer Res Treat,2007,6(1):37–48.doi:10.1177/153303460700600106.
[21] Ruarus AH,Vroomen L,Puijk RS,et al.Irreversible Electroporation in Hepatopancreaticobiliary Tumours[J].Can Assoc Radiol J,2018,69(1):38–50.doi:10.1016/j.carj.2017.10.005.
[22] Golberg A,Yarmush ML.Nonthermal irreversible electroporation:fundamentals,applications,and challenges[J].IEEE Trans Biomed Eng,2013,60(3):707–714.doi:10.1109/TBME.2013.2238672.
[23] Jiang C,Davalos RV,Bischof JC.A review of basic to clinical studies of irreversible electroporation therapy[J].IEEE Trans Biomed Eng,2015,62(1):4–20.doi:10.1109/TBME.2014.2367543.
[24] Lu DS,Raman SS,Vodopich DJ,et al.Effect of vessel size on creation of hepatic radiofrequency lesions in pigs:assessment of the "heat sink" effect[J].AJR Am J Roentgenol,2002,178(1):47–51.doi:10.2214/ajr.178.1.1780047.
[25] Long G,Bakos G,Shires PK,et al.Histological and finite element analysis of cell death due to irreversible electroporation[J].Technol Cancer Res Treat,2014,13(6):561–569.doi:10.7785/tcrtexpress.2013.600253.
[26] 胡水全,李晓勇,陈艳军,等.纳米刀消融术治疗不可切除肝门部胆管癌的安全性与疗效[J].中华肝胆外科杂志,2018,24(2):92–95.doi:10.3760/cma.j.issn.1007–8118.2018.02.006.
Hu SQ,Li XY,Chen YJ,et al.Safety and efficacy of nanoknife ablation for unresectable hilar cholangiocarcinoma[J].Chinese Journal of Hepatobiliary Surgery,2018,24(2):92–95.doi:10.3760/cma.j.issn.1007–8118.2018.02.006.
[27] Martin RC 2nd,Kwon D,Chalikonda S,et al.Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation:safety and efficacy[J].Ann Surg,2015,262(3):486–494.doi:10.1097/SLA.0000000000001441.
[28] Vogel JA,Rombouts SJ,de Rooij T,et al.Induction Chemotherapy Followed by Resection or Irreversible Electroporation in Locally Advanced Pancreatic Cancer (IMPALA):A Prospective Cohort Study[J].Ann Surg Oncol,2017,24(9):2734–2743.doi:10.1245/s10434–017–5900–9.
[29] Kluger MD,Epelboym I,Schrope BA,et al.Single-Institution Experience with Irreversible Electroporation for T4 Pancreatic Cancer:First 50 Patients[J].Ann Surg Oncol,2016,23(5):1736–1743.doi:10.1245/s10434–015–5034-x.
[30] Moris D,Machairas N,Tsilimigras DI,et al.Systematic Review of Surgical and Percutaneous Irreversible Electroporation in the Treatment of Locally Advanced Pancreatic Cancer[J].Ann Surg Oncol,2019,26(6):1657–1668.doi:10.1245/s10434–019–07261–7.
