原发性肝癌(PLC)是临床上最常见的恶性肿瘤之一,在恶性肿瘤中,其发病率高居全球第3位[1]。 其中又以亚洲和非洲地区高发[2]。原发性肝癌最多见的是肝细胞癌(HCC)。在我国,HCC是第4位最常见的恶性肿瘤和第三大的肿瘤相关致死癌症[3]。 在全球每年HCC新发患者中,超过50%者发生在中国[4]。PLC的治疗方式主要包括:手术治疗、经肝动脉化疗栓塞和局部治疗等,其中以手术治疗为主,术后并发症发生率仍较高,并且严重威胁患者生命,这使得术前准确评估肝脏储备功能尤为重要。肝脏的储备功能是指肝脏受损后机体维持正常机能的代偿能力,除了机体所需的代谢、蛋白质合成或降解、解毒功能以外的创伤修复能力和肝脏再生能力。其可作为临床上评估肝脏耐受手术方式的重要指标,也是分析病情、判断患者预后的重要指标。但是,以目前临床上常用的方法难以准确评估肝储备功能,近期由于技术的进展极大提高了评估肝储备功能的能力。本文将对目前肝功能储备评估方法进行分类概述。
1 实验室评估方法
通过常规的实验室检查,可以快速、简单地评估肝功能储备:⑴ 反映肝脏损伤的指标:丙氨酸氨基转移酶(ALT)、天门冬氨酸氨基转移酶(AST)、乳酸脱氢酶、胆碱酯酶等,其中ALT和AST不断增高可在一定程度上反映出肝细胞损害和坏死程度,而非反映肝储备功能。⑵ 反映肝脏合成指标:前白蛋白,白蛋白(ALB)、凝血酶原时间(PT)、国际标准化比值(INR)等,白蛋白仅仅在肝脏中合成,是临床上最为常用的反映肝脏合成的指标;而PT和INR则实时反映了肝功能状态。⑶ 反映肝脏转化、排泄功能指标:总胆红素(TBIL)、直接胆红素(DBIL)、总胆酸、血氨、碱性磷酸酶等;血浆中TBIL水平通常被认为是肝脏排泄功能的主要指标,因此,一旦血清TBIL水平明显升高,则表明肝功能受损较重;TBIL是组成Child-Pugh分级的重要指标,作为HCC患者术前肝储备功能评估标准广泛应用于临床,评估HCC 患者是否可行肝切除术[5-7]。但是需要注意的是,不可将HCC伴有黄疸均视为肝功能失代偿而放弃手术,部分患者是由于肿瘤压迫、胆管癌栓等阻塞胆道而引起的黄疸,有研究[8-9] 发现术前行减黄治疗,这部分患者仍能行手术治疗,且能获益;对于合并时间大于2周的梗阻性黄疸患者,血清TBIL>200 μmol/L,术前应先减黄治疗,以增强肝脏对手术的耐受能力[10]。血氨值目前在临床上很少监测,除非患者发展为肝性脑病。⑷ 反映肝间质成分的指标:透明质酸(HA)、层黏连蛋白(LN)、血清IV型胶原(C-IV)等。HA用于反映肝纤维化程度和肝窦内皮细胞清除功能,Yachida等[11]认为术前HA浓度与术后肝功能衰竭(PHLF)的发生呈正相关。但是目前任何单一的实验室检测指标均无法客观反映肝储备功能,且准确率较低。主要采取多个实验室检测指标联合评估,可在一定程度上提高其预测准确度。如白蛋白-胆红素(ALBI)评分,联合了白蛋白和胆红素两个指标,可用于预测PHLF的发生,并且可预测患者总体生存率(OS)和无复发生存率(RFS)发生情况[12]。基于ALBI,近期提出了血小板-白蛋白-总胆红素分级(PALBI)[13-14],Wu等[15]将ALBI评分、PALBI评分和肿瘤大小三者结合,可明显提高PHLF预测准确度。ALT活性/血小板计数比值(ALT-to-platelet count ratio index,APRI)可以反映肝炎活动性和肝纤维化程度,Ichikawa等[16]通过多因素分析,发现APRI是PHLF独立预测因子,并提出APRI>10是预测PHLF的临界值。近期也有学者[17]提出将APRI评分和ALBI评分相结合,能更有效预测患者术后复发及生存情况。
2 临床系统评估
2.1 Child-Pugh 评分系统
Child-Pugh评分系统是由Pugh等总结并完善了Child和Turcotte的研究成果,并于1973年发表在英国外科杂志上,此后该评分被广泛应用于临床。该评分参数是基于常规的实验室检查(表1),可以反映肝损害的已有状态和(或)肝脏的代偿现状,决定肝癌患者是否可行肝切除术,但是无法确定肝癌切除的安全范围。目前普遍接受的是,处于Child-Pugh A级的患者行肝切除术是相对安全的,不推荐Child-Pugh C级患者行肝切除术,当患者处于Child-Pugh B级时,视患者的具体情况来制定诊疗计划[5-7];但是研究[12,18]发现,即使术前肝功能Child-Pugh评分为A级的患者,其术后仍有一定的概率发生PHLF。该评分系统仍有很多不足:⑴ 由于该评分由几个参数组成,所以可能会出现同一评分等级但是实际肝功能却不相同的情况。⑵ 该评分系统纳入了腹水和肝性脑病分级,易受主观因素影响;而白蛋白、总胆红素、PT很容易受到外部因素的影响。⑶ 对于没有肝硬化患者,行肝切除术对肝功能影响相对较小,此时Child-Pugh评分系统并不适合用于评估此类患者术后情况。
表1 Child-Pugh 评分分级标准
Table1 Child-Pugh scoring system
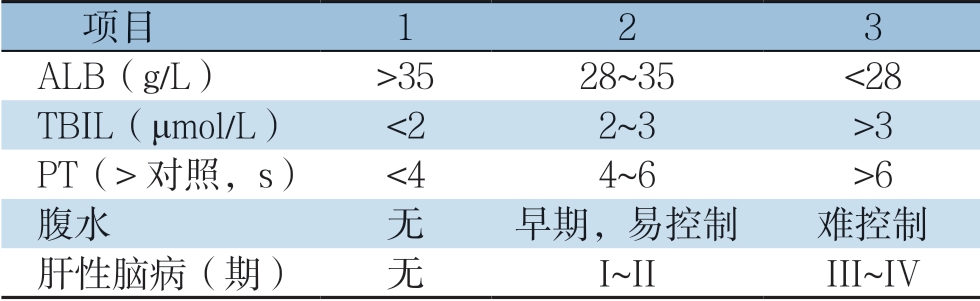
项目 123 ALB(g/L) >3528~35 <28 TBIL(μmol/L) <22~3 >3 PT(>对照,s) <44~6 >6腹水 无 早期,易控制 难控制肝性脑病(期) 无 I~II III~IV
2.2 终末期肝病模型(MELD)评分
MELD评分最初是用于预测肝癌经颈静脉肝内门体分流术术后的生存情况[19],目前MELD评分用于预测严重肝脏衰竭患者的病死率和决定终末期肝病患者是否可行肝移植及其先后顺序,也用于评估慢性肝病的严重程度和预后。该评分系统考虑了肾功能状况,能对病情的严重程度作出精细的评估,能对终末期肝病患者的病情严重程度及预后进行较为准确评估,该系统的计算公式:R=0.378×ln[TBIL(μmol/L)÷17.1]+1.12×ln(国际标准化比值)+0.957×ln[肌酐(μmol/L)÷88.4]+0.643(病因为胆汁性或酒精性取0,其他取1),结果取整数,R值越高,风险越大,其生存率越低。其被认为与肝功能损害程度相关,与Child-Pugh评分系统相似,可用于判断肝硬化患者病情进行性恶化的辅助工具[20]。由于MELD不能准确的预测行肝切除术后患者的OS,所以该评分系统应用较为局限。在随后的研究中发现,当患者MELD评分相等,但血清钠浓度不相等时,术后病死率不同。在2006年,基于MELD评分,Biggins等[21]提出了MELD-Na评分系统,MELD-Na评分=MELD+1.59×[135-Na+(mmol/L)],认为其可以更好的优化肝移植分配系统。但是也有研究[22]发现将Na+纳入MELD评分系统并没有提升其在预测肝癌合并肝硬化患者肝切除术后的急性肝衰的价值。为了更好地预测肝脏疾病的预后,基于MELD评分系统提出了多种改良的评分系统,如英国末期肝病[23](united kingdom end-stage liver disease,UKELD)、MELD/Na+(MESO)等。
3 吲哚菁绿(indocyanine green,ICG)清除试验评估
ICG 是一种水溶性荧光染料,它经静脉注入人体后,在血液中与血清蛋白结合,随血循环迅速分布到全身血管内,继之被肝细胞所摄取,在肝细胞内无结合,然后以游离形式分泌到胆汁,经肠、粪便排出体外,不参加肝、肠循环与生化转化,也不从肾脏排泄,ICG安全无毒,ICG相关不良反应罕见,但由于其含有碘成分,碘过敏或甲状腺功能亢进者慎用。ICG清除率取决于肝血流量、有功能的肝细胞量、胆道是否通畅等。在ICG清除率试验中,最能有效的评估肝脏储备功能的指标是15 min后的ICG滞留率(ICG-R15)、ICG血浆清除率(ICG-PDR)以及肝有效血流量(effective hepatic blood flow,EHBF)。过去认为,ICG-R15<10%,右半肝切除被认为是安全的;而患者 ICG-R15 在 10%~19%范围内,左半肝切除术被认为是安全的;患者ICG-R 15 在20%~29%范围内,只能切除约1/6的肝脏体积;患者ICG-R15>30%,只能行肿瘤局限性切除[24]。随着医学科学的发展,手术模式逐步转向精准外科切除,为使患者最大化获益;提出了必需功能性标准化肝体积比(ratio of essential to standard liver volume,RES)即患者必需功能性肝脏体积与标准肝脏体积的比值,正常肝脏的RES为0.2~0.25,当肝功能Child-Pugh A级时,若ICG-R15<10%,则RES≥0.4;若ICG-R 15 为10%~20%,则RES≥0.6;若ICG-R 15为21%~30%,则RES ≥0.8;若ICG-R15为31%~40%,只能行限量肝切除术;若ICG-R15>40%或肝功能Child-Pugh B级,只能行肿瘤切除术;肝功能Child-Pugh C级为肝切除术禁忌证[25]。Wang等[18]研究185例行肝切除术肝癌患者,发现ICG-R15是PHLF的独立预测因素,提出ICG-R15<7.1%行大部分肝切除(>3个肝段)是相对安全的,并认为ICG-R15比Child-Pugh评分和MELD评分能更准确用于术前肝功能储备评估。当肝癌患者行立体定向体放射治疗(stereotactic body radiation therapy,SBPT),ICG-R15不仅提供肝功能储备信息,预测术后肝损害程度;而且还可以指导肝癌患者行放射治疗时射线的最大安全剂量[26]。虽然ICG清除试验目前被认为是预测肝功能储备较为准确的定量评估方法,但是ICG的排泄试验受到很多方面的影响,例如慢性肝病的恶化将会导致肝纤维化加重[27]、侧支循环生成、肝内分流形成、胆红素急剧升高等,而这一些将会干扰肝血流,从而会影响ICG清除试验的结果。同时心力衰竭患者中,肝血流量减少及肝脏摄取ICG受到延迟,从而导致ICG滞留率提高使得读数不准确。并且ICG清除试验反映的是整个肝脏的功能,并不能准确反映某一肝段的功能,因此临床上也不能单纯依靠ICG来决定是否可行手术治疗及手术切除的肝脏范围。为此基于ICG清除试验,提出了未来肝脏血浆ICG清除率(future liver remnant plasma clearance rate of ICG,ICGK-FLR)[28]、白蛋白-吲哚菁绿(albumin-indocyanine green evaluation,ALICE)[29-30]等评估肝脏储备功能的方法。对于合并有门静脉高压的HCC患者,术前可联合ALICE评分评估患者是否可行手术治疗[31]。ICG清除试验在预测肝功能储备方面很有前景,但是该试验在非亚洲地区并没有得到广泛普及,目前仍需综合多国家多中心研究,完善其在预测肝功能储备方面的研究。
4 影像学方法评估
4.1 超声扫描技术
超声扫描是通过测量肝体积、肝实质回声以及血流情况等指标,进而评估肝脏储备功能。慢性肝病的治疗及预后很大程度上取决于肝脏纤维化的程度和进展,通过经皮肝穿刺肝组织活检传统上被认为是评估肝脏纤维化的金标准[32],但是肝活检术是一种侵入性操作、费用较高并且容易损伤正常肝脏组织,患者常常难以接受;近年来,非侵入性肝纤维化评估方法成为临床研究的热点,瞬时弹性成像(transient elastography,TE)、超声弹性成像的声辐射力脉冲成像(acoustic radiation force impulse,ARFI)、超声造影(contrast-enhanced ultrasound,CEUS)等已经成为新型的非侵入性肝纤维化评估方法。TE 是一种利用超声波测量肝组织中剪切波的传播,经转换后对肝脏硬度进行推测的一种新技术,其对肝硬化的诊断评估具有一定的应用价值,甚至有学者[33]认为该方法能部分取代传统的肝穿刺活检。将T E 与血清肝纤维化指标(如fibrosis-4等)相结合,可明显提升肝纤维化测定准确性[34-35]。ARFI应用于肝纤维化的评估目前尚处于临床研究阶段,其效果被认为与TE相当,对于测量肝纤维化有一定前景[36]。但由于传统二维超声诊断肝纤维化标准纷繁复杂、机器型号、医师主观判断等差别,使其在该方面的临床实用性欠缺[37]。CEUS不仅可以用于定性诊断肝脏局灶性病变的定性诊断,还可以用于肝脏弥漫性病变(如常见的肝纤维化、肝硬化等)的评价。CEUS定量参数间接反映疾病对肝内血流动力学的影响,可从拟合后的时间-强度曲线(time-intensity curve,TIC)中提取,主要包括肝静脉到达时间(hepatic vein arrival time,HVAT)、肝动-静脉渡越时间(hepatic artery-vein transit time,HAVTT)、门-肝静脉渡越时间(portal vein-hepatic vein transit time,PV-HVTT)等。Liu等[38]通过测量HVAT、HA-VTT,认为CEUS可作为无创诊断肝纤维化的半定量依据。CEUS诊断肝纤维化已取得一定进展,但CEUS的应用仅限于重度肝纤维化、肝硬化的诊断,对早期肝纤维化的诊断及分期尚无可靠指导意义。
4.2 X 线计算机断层摄影技术(computed tomography,CT)
随着计算机技术的发展,基于C T 图像的模拟三维肝切除技术在临床上应用逐渐成熟。利用 CT 三维成像技术不仅能够计算出解剖性肝脏体积,还可根据影像学准确对门静脉、肝动脉、肝静脉支配区域进行模拟,精确描述肿瘤与邻近血管、胆管的位置,从而准确计算出解剖性肝切除后的剩余肝体积[39](remnant liver volume,RLV),评估是否可行肝切除术,特别对于扩大半肝切除患者非常重要。目前仍普遍认为:肝脏无实质性病变,行70%~75%的肝切除被认为是安全的[40],而肝脏有实质性病变,如肝硬化、脂肪肝等,安全切除范围就被限制在40%~60%[41]。然而该方法的核心是基于肝脏体积的计算,其建立在同等大小的肝脏体积并具备相同的肝储备功能上,但在合并脂肪肝、肝硬化或既往行门静脉栓塞术(PVE)、联合肝脏离断及门脉结扎的分次肝切除术(ALPPS)的患者中,其肝脏发生了脂肪变性或者微血管改变,此时肝脏的容量和其肝脏储备功能并不对等[42]。有多项研究[43-44]表明,行PVE可使未来残余肝脏(FRL)功能增加,并且FRL功能的增加超过了FRL体积的增加。但是行ALPPS后FRL实际功能的增加和FRL体积的增加并不相符[45]。肝硬化是一种慢性肝脏疾病,会导致肝实质内正常小叶结构进行性破坏、变形,同时肝纤维化及结节再生引发肝内血管床结构受压梗阻、肝内血管阻力持续性增加[46],检测肝血流动力学改变能为评估肝硬化病情变化严重程度及肝功能储备提供更多有价值的功能影像学信息。定量评估肝硬化患者肝功能有助于动态监测病情及术前评价肝功能储备。CT灌注成像通过量化肝循环血流动力学的改变来反映肝功能损伤程度,但其辐射暴露剂量高且患者难以配合检查时的长时间屏气,因此临床应用受限[47]。动脉增强分数(arterial enhancement fraction,AEF)彩图灌注技术是动脉期绝对强化增强量与门静脉期绝对强化增加量的比值,能有效反映慢性肝病患者的血流动力学改变,能反映肝脏纤维化程度,与肝硬化严重程度密切相关[48-50],可用于肝硬化患者肝功能损伤程度和储备水平的评估。Bonekamp等[51]采用基于三期增强的AEF评估了肝纤维化及肝硬变严重程度,结果发现,正常肝脏或轻度肝纤维化、中重度肝纤维化及肝硬变3组间AEF值比较差异有统计学意义,并认为AEF是评估慢性肝病肝纤维化程度的一个无创、快速准确的指标。Rong等[52]前瞻性分析52例肝硬化患者,提出定量参数肝AEF(hepatic AEF,HAEF)、肝与脾AEF比值(HAEF/spleen AEF,H/S)与肝硬化严重程度及预后密切相关,能简单、有效地评估肝功能损伤状况。CT计算的肝脏体积是形态学水平的评估,是目前确定患者是否可以安全接受肝切除的方法,与肝储备功能有一定相关性,但是并不能准确反映肝细胞实质的储备功能,高估剩余肝脏功能(remnant liver function,RLF)会导致严重的术后并发症,而低估RLF功能会使患者错过最佳治疗方法,因此临床上常需要联合其他肝储备评估方法来提高预测肝储备功能的准确性。
4.3 磁共振扫描技术(MRI)
MRI评估肝脏储备功能主要从灌注加权成像(perfusion weighted imaging,PWI)、弥散加权成像(diffusion weighted imaging,DWI)、波谱分析((magnetic resonance spectrum,MRS)等方面进行分析。增强MRI相较于CT提供了更为准确的解剖信息,已经成为肝癌切除术前评估的一部分,但同CT容积法一样,仍然只是基于肝脏体积的评估,缺乏对肝功能情况准确评估。近年来普美显MRI作为一种新兴技术不断发展,有望成为诊断HCC及术前肝储备功能评估的一站式技术[53]。普美显MRI是将造影剂钆塞酸二钠(gadolinium ethoxybenzyl-diethylenetriaminepentaaceticacid,Gd-EOB-DTPA)经静脉注入人体内,是肝脏MRI特异性造影剂,进入人体内后大部分被肝细胞摄取,从胆管、肾脏排泄,并且排泄之前不存在生物转化,其摄取量和肝脏纤维化的严重性与肝硬化程度相关[54]。作为一种新型的磁共振造影剂,普美显能够在较大程度上反映出病灶的性质,同时该造影剂兼具非特异性细胞外对比剂与肝胆特异性对比剂的双重特性,可有效的缩短检查时间,并获得较为准确的检查结果,为临床医生提供可靠的检查数据保障[55]。普美显MRI最初用于肝癌的定性诊断,对于肝功能异常的患者,肝脏对Gd-EOB-DTPA的吸收量将会降低,因而强化程度或测量肝实质增强前后的T1和T2可判断肝实质对普美显的摄取标准,对肝功能进行评估[56]。普美显MRI的T1弛豫时间测量所得的相关参数可定量评估肝储备功能[57]。Gd-EOB-DTPA是一种顺磁性对比剂,可增加T1弛豫率,有效缩短组织的T1弛豫时间,肝纤维化会导致正常肝细胞数目的减少,阻碍了EOB的转运,从而导致了T1弛豫时间的减少,故测量Gd-EOB-DTPA增强前后肝实质的信号强度、或通过T1mapping成像获取T1弛豫时间可定量反映肝实质对Gd-EOB-DTPA的摄取能力,从而评估肝功能及肝纤维化[58-60]。将普美显MRI得到的对比增强比(contrast enhancement ratio,CER)和肝脏体积参数(如总肝体积(total liver volume,TLV)、RLV、RLV/SLV)相结合,可用于评估功能肝体积(functional liver volume,FLV)及预测PHLF的发生风险,从而反映肝储备功能[61-62]。Haimer等[63]认为普美显MRI的T1弛豫时间和信号强度(signal-intensity,SI)的相关参数与ICG-PDR存在显著相关性。并且T1弛豫时间在评估肝功能储备方面优于SI。ICG-R15>20%,是行大部分肝切除的禁忌证[25],此时测量肝脏的Gd-EOB-DTPA摂取率,可以确定患者是否可行小部分肝切除术[64]。Yoon等[65]通过前瞻性研究分析71例肝癌患者,术前使用普美显MRI预测术后残余肝储备功能,得到残余肝功能参数HEFml=肝提取分数(hepatic extraction fraction,HEF)×RLV(mL), 发现术前HEFml与ICG-R15呈负相关,而RLV和ICG-R15无相关性;认为普美显MRI不仅可以提高肝体积参数,还可以提高定性和定量的肝功能参数。行普美显MRI得到信号参数,如SIPOR(signal intensity-portal phase)、SI-HEP(signal intensity-hepatobiliary phase),能有效地对具有不同Child-Pugh评分和MELD评分的患者进行分类,可作为评估肝储备功能的潜在技术[66]。Verloh等[67]按照MELD评分为标准,提出普美显MRI能有效的对肝功能不全进行评估。基于普美显MRI得到的标准化肝功能(standardized liver function,SLF),Yamada等[68]提出肝切除安全范围公式:70×(SLF-962)/1076(%)。普美显MRI既提供准确的解剖信息,又提供了整个肝脏和各段肝脏的肝储备功能情况,但是由于其造影剂Gd-EOB-DTPA经胆道、肾排泄,所以高胆红素血症[69]及肾功能障碍会影响其准确性。
5 核医学
5.1 锝标记的去唾液酸糖蛋白类似物半乳糖化人血清清蛋白(99mTC-GSA)显像
去唾液酸糖蛋白受体(asialoglycoprotein receptor,ASGPR)是肝细胞表面的特异性受体,能专一摄取血液中的糖蛋白,其浓度和肝脏储备功能呈正相关。慢性肝炎、肝硬化和肝癌等导致肝功能受损时,该受体数量会下降。因99mTCGSA可与该受体特异性结合,通过用γ照相机使其显像,通过监测99mTC-GSA的肝脏摄取量和血液清除率,以此动态反映ASGPR浓度,评估肝储备功能,但是其检测结果可能受到散射效应、患者身体运动等因素的干扰。99mTC-GSA肝脏摄取率(liver uptake ratio at 15min,LHL15)是动态99mTC-GSA显像常用的定量指标,LHL15代表着肝细胞数量,可用于反映功能肝脏体积和肝病的严重程度,为HCC患者行肝切除术术前评估肝功能提供有用信息[70-71]。为了精确计算残余肝脏储备功能,将99mTC-GSA显像与单光子发射计算机断层显像(SPECT/CT)技术相结合,但是99mTCGSA SPECT提供的图像解剖分辨率较低[72],将其图像和CT图像相结合,以提供肿瘤位置和肿瘤与血管的关系。检测肝脏各区域GSA积累,从而精准的评估肝功能各区域分布。由99mTC-GSA SPECT/CT可得到一些参数,如肝脏摄取率(liver uptake ratio,LUR)[73]、肝脏摄取密度(liver uptake density,LUD)[47]、体表面积校正肝脏摄取值(liver uptake value corrected for body surface area,LUV)[74]和摄取指数(uptake index,UI)等。UI是基于99mTC-GSA SPECT/CT图像而计算出的指标[75],不仅可以准确反映某区域肝脏功能,还可以反映术后肝脏功能情况,不受血清胆红素等因素影响,具有安全、无创、操作简单等优点。肝脏的平均标准摂取率(mean standardized uptake value,SUVmean)由99mTC-GSA SPECT/CT计算得出,Tokorodani等[76]提出SUVmean与肝纤维化密切相关(OR=0.168,95% CI=0.048~0.435,P<0.001),并以6.7为临界值,认为SUVmean>6.7能准确预测严重肝纤维化。但是由于各个中心的仪器设备不同并且操作过程较为复杂,得到的99mTc-GSA、CT图像信息会有偏差,所以仍需要更加深入、系统地研究。
5.2 99m Tc- 甲溴苯宁(mebrofenin)肝胆显像(hepatobiliary scintigraphy,HBS)
99mTc-Mebrofenin能被肝细胞摄取并直接排泄到胆管中而不进行任何生物转化,利用γ照相机进行动态闪烁成像可使其比C T 容量法更有价值地预测PHLF的风险。但是二维平面图像缺乏在节段水平上评估肝功能的能力,遂可将其与SPECT/CT扫描的解剖信息结合,通过计算出甲溴苯宁摄取率(mebrofenin uptake rate,MUR),实现精确评估FRL的体积和功能[77]。Rassam等[53]通过比较DHCE-MRI得到的fKi(mean Ki in the future remnant liver)和99mTc-Mebrofenin HBS得到的MUR对于PHLF的预测能力,发现fKi、MUR和FRL密切相关(P=0.001和P<0.001),将fKi和M U R 结合,可以成为术前肝功能评估的一站式方法。Chapelle等[78]基于以MRI测量得到的未来剩余肝脏体积(future liver remnant volume,FLRV%)和以99mTc-Mebrofenin HBS测量得到的总体肝脏功能(total liver function,TLF),估算出未来残肝功能公式(estimated future liver remnant function,eFLRF):FLRV%×TLF,提出eFLRF<2.3%/(min.m2)是PHLF的独立预测因素。99mTc-Mebrofenin HBS可以提供功能性肝摄取和排泄信息,能够评估术前肝功能储备和RLF[79]。值得注意的是,肝脏各段功能不尽相同,特别对于肝功能受损、行PVE或ALPPS的患者,利用核成像技术对肝脏功能进行定量评估,可同时提供肝脏的解剖和功能信息[80],但是Mebrofenin与ICG相似,需从胆道排泄,故对于胆道梗阻患者,其所提供的肝储备功能信息可靠性较差[81]。
6 其他方法
利多卡因主要在肝脏内代谢,约3%以原形从肾脏排泄,注射利多卡因后监测其代谢产物单乙基二甲苯胺(monoethylglycinexylidide,MEGX)的血液浓度可以较为快捷地有效评估肝脏储备功能[82]。利多卡因代谢试验评估肝功能储备多用于肝移植供体的选择及移植术后监测移植肝脏功能情况。但是MEGX需要不断监测,受到血液中胆固醇、甘油三酯、胆色素等干扰,并且不适合于慢性肝炎患者[83-84],临床应用较少。氨基比林为肝细胞摄取药物,利用放射性核素13C或14C标记的二甲基氨基比林在肝微粒体氧化酶系P450作用下产生出CO2,测定呼出的CO2速率和呼出量,反映肝细胞受损和肝功能储备情况。正常人2 h14C排除率为7%,低于此数值表明肝储备功能下降。此类试验具有非侵入性、高安全性、高灵敏性、高特异性、可实时动态检测的优点[85];但是吸烟、使用药物、胆道梗阻时胆汁酸盐会对细胞色素P450抑制,所以在预测肝功能方面有一定局限性[86]。ABT多用于评估肝硬化患者的预后[87],用于预测肝切除的手术风险较少。
7 小结及展望
尽管目前肝癌切术后的病死率大幅度降低,但是PHLF仍然高发并且威胁患者生命,PHLF与肝硬化程度、术前肝功能状态、术中肝门阻断时间、肝切除量、术中出血量等有密切关系。术前准确评估肝储备功能能极大降低PHLF的发生,传统的评估肝功能的方法,如Child-Pugh评分、MELD评分、ICG清除试验等,对于评估肝储备功能和降低手术风险有很大意义,但是它们都只反映了整个肝脏的功能,很难用于评估各节段肝脏储备功能。CT可以制作出三维图像让外科医生模拟行肝切除术,计算RLV并且可以判断肝切除术是否安全,但是,肝脏体积并不能准确反映出肝脏功能。准确评估肝脏功能应该包括解剖数据和整个肝脏及术后剩余肝脏功能等参数。99mTc-GSA联合SPECT/CT能够提供肝脏解剖等数据,又可计算剩余肝脏功能,特别对于行ALPSS和PVE后患者的预后有独特价值,对于确定肝切除边缘及预测术后风险很有前景,但目前由于其成本较高,参数计算方法较为复杂,仍然需要更进一步研究以完善该评估方法。MRI可以提供辨析度极高的解剖图像,在引入Gd-EOB-DTPA后可同时获取肝功能信息,且该方式为无创检查方式,能够避免对患者造成不良损伤,目前看来具有广阔的临床应用前景。但每一种评估方法都存在一定的局限性,只有将多种评估方法综合起来,术前才能对HCC患者的肝储备功能进行精准评估,从而制定高度个体化的治疗方案,提高手术成功率及患者远期生存率。
[1] Torre LA,Bray F,Siegel RL,et al.Global cancer statistics,2012[J].CA Cancer J Clin,2015,65(2):87–108.doi:10.3322/caac.21262.
[2] 付艳,邢卉春.原发性肝癌的流行状况及危险因素分析[J].中国肝脏病杂志:电子版,2014,7(2):87–90.doi:10.3969/j.issn.1674– 7380.2014.02.025.
Fu Y,Xing HC.Analysis of prevalence and risk factors of primary liver cancer[J].Chinese Journal of Liver Diseases:Electronic Version,2014,7(2):87–90.doi:10.3969/j.issn.1674–7380.2014.02.025.
[3] 中华人民共和国国家卫生和计划生育委员会.原发性肝癌诊疗规范(2017年版)[J].传染病信息,2017,30(3):111–127.doi:10.3969/j.issn.1007–8134.2017.03.001.
The National Health and Family Planning Commission of the People's Republic of China.Standards for diagnosis and treatment of primary liver cancer (2017 edition)[J].Infectious Disease Information,2017,30(3):111–127.doi:10.3969/j.issn.1007–8134.2017.03.001.
[4] 张志伟,陈孝平.《原发性肝癌诊疗规范》(2017 版)解读[J].临床外科杂志,2018,26(1):5–8.doi:10.3969/j.issn.1005–6483.2018.01.001.
Zhang ZW,Chen XP.Interpretation of Standards for diagnosis and treatment of primary liver cancer (2017 edition)[J].Journal of Clinical Surgery,2018,26(1):5–8.doi:10.3969/j.issn.1005–6483.2018.01.001.
[5] European Association for the Study of the Liver.Electronic address:easloffice@easloffice.eu,European Association for the Study of the Liver.EASL Clinical Practice Guidelines:Management of hepatocellular carcinoma[J].J Hepatol,2018,69(1):182–236.doi:10.1016/j.jhep.2018.03.019.
[6] Heimbach JK,Kulik LM,Finn RS,et al.AASLD guidelines for the treatment of hepatocellular carcinoma[J].Hepatology,2018,67(1):358–380.doi:10.1002/hep.29086.
[7] Kokudo N,Takemura N,Hasegawa K,et al.Clinical practice guidelines for hepatocellular carcinoma:The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update[J].Hepatol Res,2019,49(10):1109–1113.doi:10.1111/hepr.13411.
[8] Lassen K,Coolsen MM,Slim K,et al.Guidelines for perioperative care for pancreaticoduodenectomy:Enhanced Recovery After Surgery (ERAS®) Society recommendations[J].Clin Nutr,2012,31(6):817–830.doi:10.1016/j.clnu.2012.08.011.
[9] Farges O,Regimbeau JM,Fuks D,et al.Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma[J].Br J Surg,2013,100(2):274–283.doi:10.1002/bjs.8950.
[10] Saxena P,Kumbhari V,Zein ME,et al.Preoperative biliary drainage[J].Dig Endosc,2015,27(2):265–277.doi:10.1111/den.12394.
[11] Yachida S,Wakabayashi H,Okano K,et al.Prediction of posthepatectomy hepatic functional reserve by serum hyaluronate[J].Br J Surg,2009,96(5):501–508.doi:10.1002/bjs.6560.
[12] Wang YY,Zhong JH,Su ZY,et al.Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma[J].Br J Surg,2016,103(6):725–734.doi:10.1002/bjs.10095.
[13] Liu PH,Hsu CY,Hsia CY,et al.ALBI and PALBI grade predict survival for HCC across treatment modalities and BCLC stages in the MELD Era[J].J Gastroenterol Hepatol,2017,32(4):879–886.doi:10.1111/jgh.13608.
[14] Luo HM,Zhao SZ,Li C,et al.Preoperative platelet-albuminbilirubin grades predict the prognosis of patients with hepatitis B virus-related hepatocellular carcinoma after liver resection:A retrospective study[J].Medicine (Baltimore),2018,97(12):e226.doi:10.1097/MD.0000000000010226.
[15] Wu B,Hu X,Jin H,et al.Albumin-bilirubin and plateletalbumin-bilirubin grades for hepatitis B-associated hepatocellular carcinoma in Child-Pugh A patients treated with radical surgery:A retrospective observational study[J].Medicine (Baltimore),2019,98(43):e17394.doi:10.1097/MD.0000000000017394.
[16] Ichikawa T,Uenishi T,Takemura S,et al.A simple,noninvasively determined index predicting hepatic failure following liver resection for hepatocellular carcinoma[J].J Hepatobiliary Pancreat Surg,2009,16(1):42–48.doi:10.1007/s00534–008–0003–4.
[17] Luo H,Li C,Chen L.Preoperative albumin-bilirubin grade combined with aspartate aminotransferase-to-platelet count ratio index predict outcomes of patients with hepatocellular carcinoma within Milan criteria after liver resection[J].Biosci Trends,2019,13(2):176–181.doi:10.5582/bst.2019.01088.
[18] Wang YY,Zhao XH,Ma L,et al.Comparison of the ability of Child-Pugh score,MELD score,and ICG-R15 to assess preoperative hepatic functional reserve in patients with hepatocellular carcinoma[J].J Surg Oncol,2018,118(3):440–445.doi:10.1002/jso.25184.
[19] Malinchoc M,Kamath PS,Gordon FD,et al.A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts[J].Hepatology,2000,31(4):864–871.doi:10.1053/he.2000.5852。
[20] Botta F,Giannini E,Romagnoli P,et al.MELD scoring system is useful for predicting prognosis in patients with liver cirrhosis and is correlated with residual liver function:a European study[J].Gut,2003,52(1):134–139.doi:10.1136/gut.52.1.134.
[21] Biggins SW,Kim WR,Terrault NA,et al.Evidence-based incorporation of serum sodium concentration into MELD[J].Gastroenterology,2006,130(6):1652–1660.doi:10.1053/j.gastro.2006.02.010.
[22] Manka P,Bechmann LP,Tacke F,et al.Serum sodium based modification of the MELD does not improve prediction of outcome in acute liver failure[J].BMC Gastroenterol,2013,13:58.doi:10.1186/1471–230X-13–58.
[23] Barber K,Madden S,Allen J,et al.Elective liver transplant list mortality:development of a United Kingdom end-stage liver disease score[J].Transplantation,2011,92(4):469–476.doi:10.1097/TP.0b013e318225db4d.
[24] Imamura H,Sano K,Sugawara Y,et al.Assessment of hepatic reserve for indication of hepatic resection:decision tree incorporating indocyanine green test[J].J Hepatobiliary Pancreat Surg,2005,12(1):16–22.doi:10.1007/s00534–004–0965–9.
[25] 中国研究型医院学会肝胆胰外科专业委员会.精准肝切除术专家共识[J].中华消化外科杂志,2017,16(9):883–893.doi:10.3760/cma.j.issn.1673–9752.2017.09.001.
Society for Hepatopancreatobiliary Surgery of Chinese Research Hospital Association.Expert consensus on presicion liver resection[J].Chinese Journal of Digestive Surgery,2017,16(9):883–893.doi:10.3760/cma.j.issn.1673–9752.2017.09.001.
[26] Suresh K,Owen D,Bazzi L,et al.Using Indocyanine Green Extraction to Predict Liver Function After Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma[J].Int J Radiat Oncol Biol Phys,2018,100(1):131–137.doi:10.1016/j.ijrobp.2017.09.032.
[27] Danin PE,Anty R,Patouraux S,et al.Non-invasive Evaluation of NAFLD with Indocyanine Green Clearance Test:a Preliminary Study in Morbidly Obese Patients Undergoing Bariatric Surgery[J].Obes Surg,2018,28(3):735–742.doi:10.1007/s11695–017–2914–0.
[28] Yokoyama Y,Nishio H,Ebata T,et al.Value of indocyanine green clearance of the future liver remnant in predicting outcome after resection for biliary cancer[J].Br J Surg,2010,97(8):1260–1268.doi:10.1002/bjs.7084.
[29] Kokudo T,Hasegawa K,Amikura K,et al.Assessment of Preoperative Liver Function in Patients with Hepatocellular Carcinoma - The Albumin-Indocyanine Green Evaluation (ALICE) Grade[J].PLoS One,2016,11(7):e159530.doi:10.1371/journal.pone.0159530.
[30] Honmyo N,Kobayashi T,Hamaoka M,et al.Comparison of new prognostic systems for patients with resectable hepatocellular carcinoma:Albumin-Bilirubin grade and Albumin-Indocyanine Green Evaluation grade[J].Hepatol Res,2019,49(10):1218–1226.doi:10.1111/hepr.13393.
[31] Shirata C,Kokudo T,Arita J,et al.Albumin-Indocyanine Green Evaluation (ALICE) grade combined with portal hypertension to predict post-hepatectomy liver failure[J].Hepatol Res,2019,49(8):942–949.doi:10.1111/hepr.13327.
[32] Bravo AA,Sheth SG,Chopra S.Liver biopsy[J].N Engl J Med,2001,344(7):495–500.doi:10.1056/NEJM200102153440706.
[33] Hashemi SA,Alavian SM,Gholami-Fesharaki M.Assessment of transient elastography (FibroScan) for diagnosis of fibrosis in non-alcoholic fatty liver disease:A systematic review and metaanalysis[J].Caspian J Intern Med,2016,7(4):242–252.
[34] Boursier J,Guillaume M,Leroy V,et al.New sequential combinations of non-invasive fibrosis tests provide an accurate diagnosis of advanced fibrosis in NAFLD[J].J Hepatol,2019,71(2):389–396.doi:10.1016/j.jhep.2019.04.020.
[35] Boursier J,Zarski J P,de Ledinghen V,et al.Determination of reliability criteria for liver stiffness evaluation by transient elastography[J].Hepatology,2013,57(3):1182–1191.doi:10.1002/hep.25993.
[36] Chen SH,Lai HC,Chiang IP,et al.Performance of Acoustic Radiation Force Impulse Elastography for Staging Liver Fibrosis in Patients with Chronic Hepatitis C after Viral Eradication[J].Clin Infect Dis,2020,70(1):114–122.doi:10.1093/cid/ciz161.
[37] Lin YS.Ultrasound Evaluation of Liver Fibrosis[J].J Med Ultrasound,2017,25(3):127–129.doi:10.1016/j.jmu.2017.04.001.
[38] Liu H,Liu J,Zhang Y,et al.Contrast-enhanced ultrasound and computerized tomography perfusion imaging of a liver fibrosis-early cirrhosis in dogs[J].J Gastroenterol Hepatol,2016,31(9):1604–1610.doi:10.1111/jgh.13320.
[39] Tu R,Xia L P,Yu AL,et al.Assessment of hepatic functional reserve by cirrhosis grading and liver volume measurement using CT[J].World J Gastroenterol,2007,13(29):3956–3961.doi:10.3748/wjg.v13.i29.3956.
[40] Yigitler C,Farges O,Kianmanesh R,et al.The small remnant liver after major liver resection:how common and how relevant?[J].Liver Transpl,2003,9(9):S18–25.doi:10.1053/jlts.2003.50194.
[41] Shoup M,Gonen M,D'Angelica M,et al.Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resectio[J].J Gastrointest Surg,2003,7(3):325–330.doi:10.1016/s1091–255x(02)00370–0.
[42] Martel G,Cieslak KP,Huang R,et al.Comparison of techniques for volumetric analysis of the future liver remnant:implications for major hepatic resections[J].HPB (Oxford),2015,17(12):1051–1057.doi:10.1111/hpb.12480.
[43] Meier RP,Toso C,Terraz S,et al.Improved liver function after portal vein embolization and an elective right hepatectomy[J].HPB (Oxford),2015,17(11):1009–1018.doi:10.1111/hpb.12501.
[44] Cieslak KP,Huisman F,Bais T,et al.Future remnant liver function as predictive factor for the hypertrophy response after portal vein embolization[J].Surgery,2017,162(1):37–47.doi:10.1016/j.surg.2016.12.031.
[45] Tanaka K,Matsuo K,Murakami T,et al.Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS):Shortterm outcome,functional changes in the future liver remnant,and tumor growth activity[J].Eur J Surg Oncol,2015,41(4):506–512.doi:10.1016/j.ejso.2015.01.031.
[46] Tsochatzis EA,Bosch J,Burroughs AK.Liver cirrhosis[J].Lancet,2014,383(9930):1749–1761.doi:10.1016/S0140–6736(14)60121–5.
[47] Geisel D,Lüdemann L,Hamm B,et al.Imaging-Based Liver Function Tests--Past,Present and Future[J].Rofo,2015,187(10):863–871.doi:10.1055/s-0035–1553306.
[48] Bonekamp D,Bonekamp S,Ou HY,et al.Assessing liver fibrosis:comparison of arterial enhancement fraction and diffusion-weighted imaging[J].J Magn Reson Imaging,2014,40(5):1137–1146.doi:10.1002/jmri.24472.
[49] Park M,Chung YE,Kim KA,et al.Added value of arterial enhancement fraction color maps for the characterization of small hepatic low-attenuating lesions in patients with colorectal cancer[J].PLoS One,2015,10(2):e114819.doi:10.1371/journal.pone.0114819.
[50] Huber AT,Schuster F,Ebner L,et al.Hepatocellular Carcinoma Screening With Computed Tomography Using the Arterial Enhancement Fraction With Radiologic-Pathologic Correlation[J].Invest Radiol,2016,51(1):25–32.doi:10.1097/RLI.0000000000000201.
[51] Bonekamp D,Bonekamp S,Geiger B,et al.An elevated arterial enhancement fraction is associated with clinical and imaging indices of liver fibrosis and cirrhosis[J].J Comput Assist Tomogr,2012,36(6):681–689.doi:10.1097/RCT.0b013e3182702ee3.
[52] 容鹏飞,冯智超,郭睿,等.CT动脉增强分数评估肝硬化患者肝功能水平[J].中南大学学报:医学版,2019,44(5):469–476.doi:10.11817/j.issn.1672–7347.2019.05.001.
Rong PF,Feng ZC,Guo R,et al.CT-based estimation of liver function using arterial enhancement fraction in liver cirrhosis patients[J].Journal of Central South University:Medical Science,2019,44(5):469–476.doi:10.11817/j.issn.1672–7347.2019.05.001.
[53] Rassam F,Zhang T,Cieslak KP,et al.Comparison between dynamic gadoxetate-enhanced MRI and (99m)Tc-mebrofenin hepatobiliary scintigraphy with SPECT for quantitative assessment of liver function[J].Eur Radiol,2019,29(9):5063–5072.doi:10.1007/s00330–019–06029–7.
[54] Verloh N,Utpatel K,Haimerl M,et al.Liver fibrosis and Gd-EOBDTPA-enhanced MRI:A histopathologic correlation[J].Sci Rep,2015,5:15408.doi:10.1038/srep15408.
[55] 周继海,李军山.普美显磁共振增强成像在鉴别肝硬化结节与小肝癌的临床应用探讨[J].影像研究与医学应用,2018,2(9):68–69.doi:10.3969/j.issn.2096–3807.2018.09.039.
Zhou JH,Li JS.Analysis of clinical application of Gd-EOBDTPA MRI in differeential diagnosis of liver cirrhosis nodule and small liver cancer[J].Journal of Imaging Research and Medical Applications,2018,2(9):68–69.doi:10.3969/j.issn.2096–3807.2018.09.039.
[56] Nilsson H,Blomqvist L,Douglas L,et al.Assessment of liver function in primary biliary cirrhosis using Gd-EOB-DTPAenhanced liver MRI[J].HPB (Oxford),2010,12(8):567–576.doi:10.1111/j.1477–2574.2010.00223.x.
[57] Katsube T,Okada M,Kumano S,et al.Estimation of liver function using T1 mapping on Gd-EOB-DTPA-enhanced magnetic resonance imaging[J].Invest Radiol,2011,46(4):277–283.doi:10.1097/RLI.0b013e318200f67d.
[58] Haimerl M,Utpatel K,Verloh N,et al.Gd-EOB-DTPA-enhanced MR relaxometry for the detection and staging of liver fibrosis[J].Sci Rep,2017,7:41429.doi:10.1038/srep41429.
[59] Pan S,Wang X Q,Guo Q Y.Quantitative assessment of hepatic fibrosis in chronic hepatitis B and C:T1 mapping on Gd-EOBDTPA-enhanced liver magnetic resonance imaging[J].World J Gastroenterol,2018,24(18):2024–2035.doi:10.3748/wjg.v24.i18.2024.
[60] Sheng RF,Wang HQ,Yang L,et al.Assessment of liver fibrosis using T1 mapping on Gd-EOB-DTPA-enhanced magnetic resonance[J].Dig Liver Dis,2017,49(7):789–795.doi:10.1016/j.dld.2017.02.006.
[61] Chuang YH,Ou HY,Lazo MZ,et al.Predicting post-hepatectomy liver failure by combined volumetric,functional MR image and laboratory analysis[J].Liver Int,2018,38(5):868–874.doi:10.1111/liv.13608.
[62] Kudo M,Gotohda N,Sugimoto M,et al.Evaluation of liver function using gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid enhanced magnetic resonance imaging based on a three-dimensional volumetric analysis system[J].Hepatol Int,2018,12(4):368–376.doi:10.1007/s12072–018–9874-x.
[63] Haimerl M,Verloh N,Zeman F,et al.Gd-EOB-DTPA-enhanced MRI for evaluation of liver function:Comparison between signalintensity-based indices and T1 relaxometry[J].Sci Rep,2017,7:43347.doi:10.1038/srep43347.
[64] Yoon JH,Lee JM,Kang HJ,et al.Quantitative Assessment of Liver Function by Using Gadoxetic Acid-enhanced MRI:Hepatocyte Uptake Ratio[J].Radiology,2019,290(1):125–133.doi:10.1148/radiol.2018180753.
[65] Yoon JH,Choi JI,Jeong YY,et al.Pre-treatment estimation of future remnant liver function using gadoxetic acid MRI in patients with HCC[J].J Hepatol,2016,65(6):1155–1162.doi:10.1016/j.jhep.2016.07.024.
[66] Ippolito D,Famularo S,Giani A,et al.Estimating liver function in a large cirrhotic cohort:Signal intensity of gadolinium-ethoxybenzyldiethylenetriamine penta-acetic acid-enhanced MRI[J].Dig Liver Dis,2019,51(10):1438–1445.doi:10.1016/j.dld.2019.04.009.
[67] Verloh N,Utpatel K,Zeman F,et al.Diagnostic performance of Gd-EOB-DTPA-enhanced MRI for evaluation of liver dysfunction:a multivariable analysis of 3T MRI sequences[J].Oncotarget,2018,9(91):36371–36378.doi:10.18632/oncotarget.26368.
[68] Yamada S,Shimada M,Morine Y,et al.A new formula to calculate the resection limit in hepatectomy based on Gd-EOB-DTPAenhanced magnetic resonance imaging[J].PLoS One,2019,14(1):e0210579.doi:10.1371/journal.pone.0210579.
[69] Okada M,Murakami T,Kuwatsuru R,et al.Biochemical and Clinical Predictive Approach and Time Point Analysis of Hepatobiliary Phase Liver Enhancement on Gd-EOB-DTPAenhanced MR Images:A Multicenter Study[J].Radiology,2016,281(2):474–483.doi:10.1148/radiol.2016151061.
[70] Nanashima A,Abo T,Kudo T,et al.Usefulness of examining hepatic functional volume using technetium-99m galactosyl serum albumin scintigraphy in hepatocellular carcinoma[J].Nucl Med Commun,2013,34(5):478–488.doi:10.1097/MNM.0b013e32835f945f.
[71] Hasegawa D,Onishi H.Evaluation of Accuracy for Quantitative Predictor of Hepatic Functional Reserve Using Planar and SPECT Images in the 99mTc-GSA Scintigraphy[J].Nihon Hoshasen Gijutsu Gakkai Zasshi,2017,73(10):1055–1060.doi:10.6009/jjrt.2017_JSRT_73.10.1055.
[72] Sumiyoshi T,Shima Y,Tokorodani R,et al.CT/99mTc-GSA SPECT fusion images demonstrate functional differences between the liver lobes[J].World J Gastroenterol,2013,19(21):3217–3225.doi:10.3748/wjg.v19.i21.3217.
[73] Onodera Y,Takahashi K,Togashi T,et al.Clinical assessment of hepatic functional reserve using 99mTc DTPA galactosyl human serum albumin SPECT to prognosticate chronic hepatic diseases--validation of the use of SPECT and a new indicator[J].Ann Nucl Med,2003,17(3):181–188.doi:10.1007/bf02990020.
[74] Yoshida M,Shiraishi S,Sakaguchi F,et al.Fused 99m-Tc-GSA SPECT/CT imaging for the preoperative evaluation of postoperative liver function:can the liver uptake index predict postoperative hepatic functional reserve?[J].Jpn J Radiol,2012,30(3):255–262.doi:10.1007/s11604–011–0041–8.
[75] Mao Y,Du S,Ba J,et al.Using Dynamic 99mT c-GSA SPECT/CT fusion images for hepatectomy planning and postoperative liver failure prediction[J].Ann Surg Oncol,2015,22(4):1301–1307.doi:10.1245/s10434–014–4117–4.
[76] Tokorodani R,Sumiyoshi T,Okabayashi T,et al.Liver fibrosis assessment using 99mTc-GSA SPECT/CT fusion imaging[J].Jpn J Radiol,2019,37(4):315–320.doi:10.1007/s11604–019–00810-w.
[77] de Graaf W,van Lienden KP,van Gulik TM,et al.(99m)Tcmebrofenin hepatobiliary scintigraphy with SPECT for the assessment of hepatic function and liver functional volume before partial hepatectomy[J].J Nucl Med,2010,51(2):229–236.doi:10.2967/jnumed.109.069724.
[78] Chapelle T,Op De Beeck B,Huyghe I,et al.Future remnant liver function estimated by combining liver volumetry on magnetic resonance imaging with total liver function on (99m)Tc-mebrofenin hepatobiliary scintigraphy:can this tool predict post-hepatectomy liver failure?[J].HPB (Oxford),2016,18(6):494–503.doi:10.1016/j.hpb.2015.08.002.
[79] Olthof PB,Coelen RJS,Bennink RJ,et al.99mTc-mebrofenin hepatobiliary scintigraphy predicts liver failure following major liver resection for perihilar cholangiocarcinoma[J].HPB (Oxford),2017,19(10):850–858.doi:10.1016/j.hpb.2017.05.007.
[80] Gupta M,Choudhury PS,Singh S,et al.Liver Functional Volumetry by Tc-99m Mebrofenin Hepatobiliary Scintigraphy before Major Liver Resection:A Game Changer[J].Indian J Nucl Med,2018,33(4):277–283.doi:10.4103/ijnm.IJNM_72_18.
[81] de Graaf W,Häusler S,Heger M,et al.Transporters involved in the hepatic uptake of (99m)Tc-mebrofenin and indocyanine green[J].J Hepatol,2011,54(4):738–745.doi:10.1016/j.jhep.2010.07.047.
[82] Ben Said D,Ben Ali R,Ferchichi H,et al.Lidocaine test for easier and less time consuming assessment of liver function in several hepatic injury models[J].Hepatol Int,2011,5(4):941–948.doi:10.1007/s12072–011–9270–2.
[83] Ercolani G,Grazi GL,Callivà R,et al.The lidocaine (MEGX) test as an index of hepatic function:its clinical usefulness in liver surgery[J].Surgery,2000,127(4):464–471.doi:10.1067/msy.2000.104743.
[84] Lorf T,Schnitzbauer AA,Schaefers SK,et al.Prognostic value of the monoethylglycinexylidide (MEGX)-test prior to liver resection[J].Hepatogastroenterology,2008,55(82–83):539–543.
[85] Gorowska-Kowolik K,Chobot A,Kwiecien J.13C Methacetin Breath Test for Assessment of Microsomal Liver Function:Methodology and Clinical Application[J].Gastroenterol Res Pract,2017,2017:7397840.doi:10.1155/2017/7397840.
[86] Van Vlierberghe H,Van Durme F,Verdievel H,et al.Influence of low-dose oral contraceptives,alcohol,and grapefruit on[J].Dig Dis Sci,2001,46(1):133–139.doi:10.1023/a:1005618126997.
[87] Lebossé F,Guillaud O,Forestier J,et al.Prognostic Value of Metabolic Liver Function Tests:a Study on 711 Cirrhotic Patients[J].J Gastrointestin Liver Dis,2016,25(3):337–343.doi:10.15403/jgld.2014.1121.253.lft.
