乳腺癌是女性最常见的恶性肿瘤[1-2]。HER-2阳性乳腺癌,特别是局部晚期年轻患者,生存时间短,治疗相对棘手,病死率高[3-4],尤其值得临床医生关注。国内外指南均推荐HER-2阳性晚期乳腺癌患者应用曲妥珠单抗联合紫杉醇,对于年轻局部晚期乳腺癌患者,由于年龄这个决定预后的独立因素,曲妥珠单抗联合化疗药物可获得更高的临床缓解率,因此,临床上建议应用PCH方案(曲妥珠单抗+紫杉醇+卡铂)治疗HER-2阳性晚期年轻乳腺癌患者[5-8]。由于应用紫杉醇需要聚氧乙烯蓖麻油作为助溶剂,因此会导致不同程度的过敏反应,骨髓抑制及外周神经毒性。白蛋白结合型紫杉醇是紫杉醇与白蛋白结合形成的纳米颗粒,疗效较普通型紫杉醇高,且毒副反应较轻[9-11],笔者尝试应用改良的PCH方案(曲妥珠单抗+白蛋白结合型紫杉醇+卡铂)治疗首诊HER-2阳性晚期年轻乳腺癌患者行新辅助治疗,结果表明,疗效确切,不良反应可耐受,现报告如下。
1 资料与方法
1.1 一般资料
选取我院2016年6月—2018年12月收治的HER-2阳性局部晚期乳腺癌患者进行前瞻性研究。将患者采用随机数字法分为两组,分别应用PCH方案(PCH组)与改良PCH方案(改良PCH组)行新辅助化疗。纳入标准:⑴ 2016年6月—2018年1 2月本院收治的HER-2阳性局部晚期年轻乳腺癌患者(T2~3,N2/N3,M0;T4,任何N,M0);⑵ 病理证实为乳腺癌,免疫组织化学检查示HER-2(+++)或FISH检测HER-2扩增;⑶年龄18~40岁;⑷ 至少有1个可测量的病灶;⑸ 美国东部肿瘤协作组(Eastern Cooperative Oncology Group,ECOG)体能状态评分0~1分;⑹ 预计生存期>3个月;⑺ 机体各器官功能基本正常,血常规、肝肾功能及心电图基本正常。排除标准:⑴ 机体一般情况较差,不能耐受化疗者;⑵年龄>40岁;⑶ 妊娠、哺乳期妇女;⑷ 心功能下降,左室射血分数<55%;⑸ 合并其他肿瘤患者。本研究患者在接受治疗前均签署知情同意书,得到徐州医科大学附属宿迁医院伦理委员会的批准(编号:sqyyll2016019)。
1.2 治疗方案
改良PCH组:白蛋白结合型紫杉醇100 mg/m2静脉注射,卡铂AUC-2静脉注射,第1、8、15天,28 d为1周期或260 mg/m2静脉注射,卡铂AUC-6静脉注射,第1天,21 d为1周期。PCH组:溶剂型紫杉醇80 mg/m2静脉注射,卡铂AUC-2静脉注射,第1,8,15天,28 d为1周期或175 mg/m2静脉注射,卡铂AUC-6静脉注射,第1天,21 d为1周期。曲妥珠单抗(上海罗氏制药有限公司,国药准字J20160033),白蛋白结合型紫杉醇(美国塞尔基因公司,H20130650;国药准字H20183044,石药集团欧意药业),溶剂型紫杉醇注射液(扬子江药业集团有限公司,国药准字H20110470),卡铂(齐鲁制药有限公司,国药准字H20020284)。曲妥珠单抗的应用为3周方案(首次8 mg/kg,后每次6 mg/kg)或每周方案(首次4 mg/kg,后每次2 mg/kg),共化疗6个周期,新辅助治疗结束后,评估患者一般情况后,予以行改良根治术,术后加做放疗,曲妥珠单抗治疗1年,治疗过程中出现疾病进展、患者拒绝或无法耐受终止化疗。
1.3 近期疗效和不良反应的评价
每2个化疗周期评价近期疗效。根据实体瘤疗效评价标准1.0(Response Evaluation Criteria in Solid Tumors 1.0,RECIST 1.0)将近期疗效分为完全缓解(complete response,CR)、部分缓解(partial response,PR)、疾病稳定(stable disease,SD)和疾病进展(progressive disease,PD)。CR又分为病理完全缓解(pCR)和临床完全缓解(cCR),pCR为乳腺原发灶肿瘤完全消失,镜下未见浸润癌残留,但可含有DCIS。客观缓解率(objective response rate,ORR)=(CR+PR)/总例数×100%。化疗期间及化疗后至少每周复查1次血常规和肝肾功能,每2周期对病灶行影像学评估。放化疗结束后定期复查乳腺肿瘤指标、胸部CT、腹部彩超及骨扫描等,观察患者有无局部或远处复发转移情况。不良反应按美国国立癌症研究所不良反应事件通用术语标准3.0(National Cancer Institute Common Terminology Criteria for Adverse Events,NCI-CTC 3.0)进行评价,分为0~4度。
1.4 随访和生存分析
采用门诊或电话方式对患者进行随访,随访截止日期为2019年12月1日,随访时间为12~42个月,中位随访时间为38个月,无进展生存(progression free survival,PFS)定义为从化疗开始日至PD或任何原因所致死亡的时间。总生存(overall survival,OS)计算从化疗开始之日起至死亡或末次随访之日止。
1.5 统计学处理
采用SPSS 15.0统计软件进行数据分析,应用Kolmogorov-Smirnov检验检测计量资料正态分布情况,应用F检验进行方差齐性分析;符合正态分布的呈正态分布的计量资料以均数±标准差( ±s)表示,组间比较采用独立样本t 检验;计数资料比较采用χ2检验;两组患者的生存分析采用Logrank法比较,采用Kaplan-Meier法绘制生存曲线,P<0.05为差异有统计学意义。
±s)表示,组间比较采用独立样本t 检验;计数资料比较采用χ2检验;两组患者的生存分析采用Logrank法比较,采用Kaplan-Meier法绘制生存曲线,P<0.05为差异有统计学意义。
2 结 果
2.1 入组患者的基本情况
本研究最终纳入62例患者,其中PCH组30例,改良PCH组32例;均为绝经前女性患者;年龄18~40岁,平均31.7岁;术前穿刺病理证实为HER-2阳性乳腺癌,IIIA期17例,IIIB期25例,IIIC期20例。其中浸润性导管癌55例,浸润性小叶癌7例。所有新辅助治疗后均行乳腺癌改良根治术,术后切除标本均行免疫组化检测。两组患者在年龄、肿瘤大小、淋巴结转移、TNM分期、病理类型、ECOG评分等情况基本相似,差异均无统计学意义(均P>0.05)(表1)。
2.2 两组患者6个周期化疗后疗效及生存分析
PCH组患者有效率为100%,其中CR 6例,pCR 2例,PR 22例。改良PCH组患者有效率为100%,其中CR 14例,pCR 8例,PR 10例。统计学分析显示,改良PCH组cCR明显高于PCH组(43.8% vs.20.0%,χ2=3.997,P=0.046),pCR也明显高于PCH组(25.0% vs.6.7%,χ2=4.098,P=0.043)。
表1 两组患者临床病理特征的比较
Table1 Comparison of the clinicopathologic characteristics between the two groups of patients
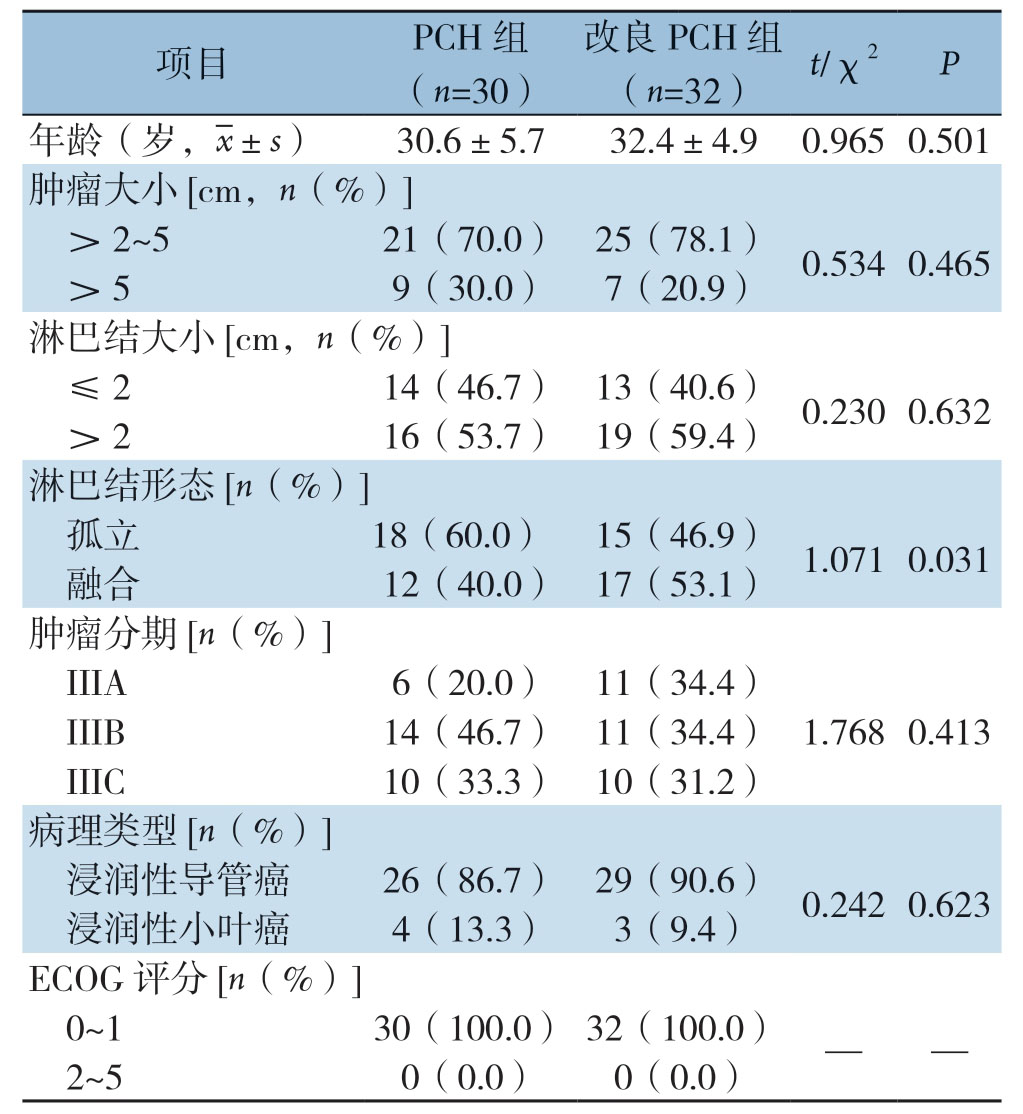
项目 PCH组(n=30)改良PCH组(n=32) t/χ2 P年龄(岁,images/BZ_82_483_743_511_786.png±s) 30.6±5.7 32.4±4.9 0.965 0.501肿瘤大小[cm,n(%)]>2~5 21(70.0) 25(78.1) 0.534 0.465>5 9(30.0) 7(20.9)淋巴结大小[cm,n(%)]≤2 14(46.7) 13(40.6) 0.230 0.632>2 16(53.7) 19(59.4)淋巴结形态[n(%)]孤立 18(60.0) 15(46.9) 1.071 0.031融合 12(40.0) 17(53.1)肿瘤分期[n(%)]IIIA 6(20.0) 11(34.4)IIIB 14(46.7) 11(34.4) 1.768 0.413 IIIC 10(33.3) 10(31.2)病理类型[n(%)]浸润性导管癌 26(86.7) 29(90.6) 0.242 0.623浸润性小叶癌 4(13.3) 3(9.4)ECOG 评分[n(%)]0~1 30(100.0) 32(100.0) — —2~5 0(0.0) 0(0.0)
2.3 两组不良反应情况
所有患者治疗期间未出现治疗相关死亡事件。紫杉类药物的主要不良反应是过敏反应,骨髓抑制,周围神经毒性反应。本研究中,改良PCH组患者中性粒细胞减少发生率为31.3%(10/32),而PCH组为60%(18/30),两者差异具有统计学意义(χ2=5.168,P=0.023);改良PCH组患者中周围感觉神经毒性发生率为40.6%(14/32),表现为手足麻木和刺痛,而PCH组的发生率为20.0%(6/30),差异有统计学意义(χ2=3.997,P=0.046),两组患者均未发生3~4度的神经毒性反应。其他常见不良反应,如恶心呕吐、脱发、皮疹等,两组间比较差异无统计学意义(均P>0.05)(表2)。另外,两组患者治疗期间心功能测定均在正常范围内。
表2 两组患者不良反应发生情况[n(%)]
Table2 Adverse reactions in the two groups of patients [n (%)]
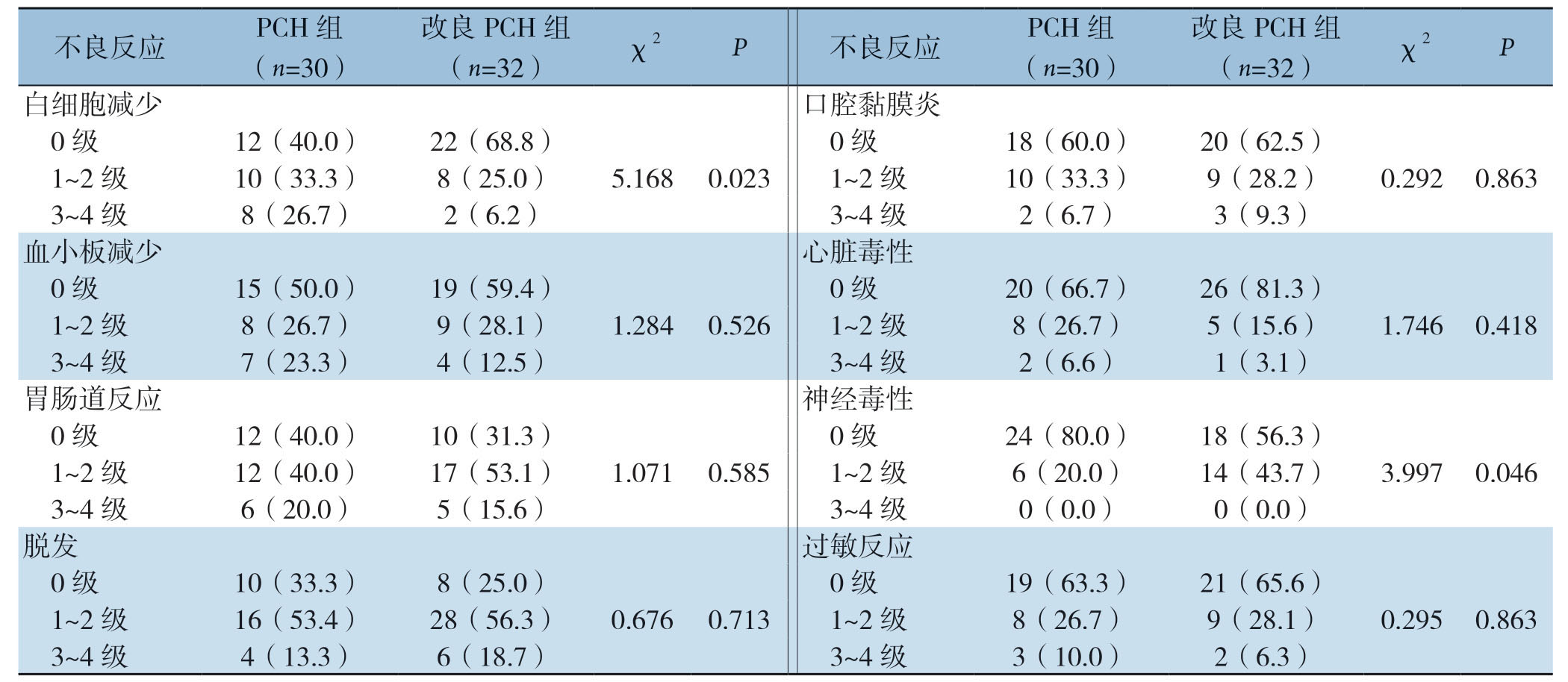
不良反应 PCH组(n=30)改良PCH组(n=32) χ2 P 不良反应 PCH组(n=30)改良PCH组(n=32) χ2 P白细胞减少 口腔黏膜炎0级 12(40.0) 22(68.8) 0级 18(60.0) 20(62.5)1~2级 10(33.3) 8(25.0) 5.168 0.023 1~2级 10(33.3) 9(28.2) 0.292 0.863 3~4级 8(26.7) 2(6.2) 3~4级 2(6.7) 3(9.3)血小板减少 心脏毒性0级 15(50.0) 19(59.4) 0级 20(66.7) 26(81.3)1~2级 8(26.7) 9(28.1) 1.284 0.526 1~2级 8(26.7) 5(15.6) 1.746 0.418 3~4级 7(23.3) 4(12.5) 3~4级 2(6.6) 1(3.1)胃肠道反应 神经毒性0级 12(40.0) 10(31.3) 0级 24(80.0) 18(56.3)1~2级 12(40.0) 17(53.1) 1.071 0.585 1~2级 6(20.0) 14(43.7) 3.997 0.046 3~4级 6(20.0) 5(15.6) 3~4级 0(0.0) 0(0.0)脱发 过敏反应0级 10(33.3) 8(25.0) 0级 19(63.3) 21(65.6)1~2级 16(53.4) 28(56.3) 0.676 0.713 1~2级 8(26.7) 9(28.1) 0.295 0.863 3~4级 4(13.3) 6(18.7) 3~4级 3(10.0) 2(6.3)
2.4 两组术后生存情况
改良PCH组中位PFS时间为13.1个月,中位OS时间为35.4个月。PCH组中位PFS时间为7.8个月,中位OS时间为21.6个月,统计学分析显示,改良PCH组较PCH组中位PFS时间及OS时间均明显延长(χ2=8.302、8.557,P=0.005、0.004)(表3)(图1)。
表3 两组患者术后生存比较(月)
Table3 Comparison of the postoperative survivals between the two groups of patients (month)
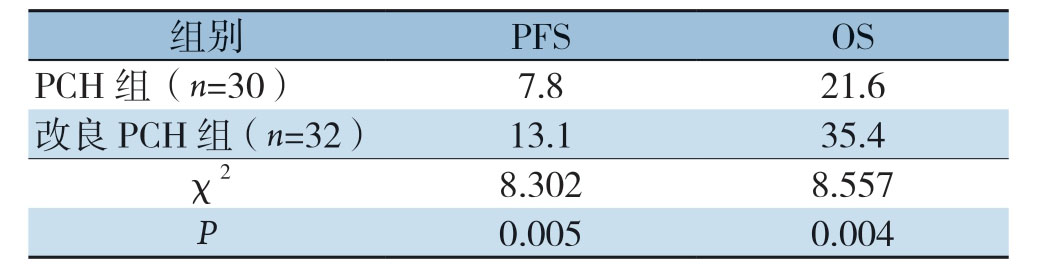
组别 PFS OS PCH组(n=30) 7.8 21.6改良PCH组(n=32) 13.1 35.4 χ2 8.302 8.557 P 0.005 0.004

图1 两组患者生存曲线比较 A:OS曲线;B:PFS曲线
Figure1 Comparison of the survival curves between the two groups of patients A:OS curves; B:PFS curves
3 讨 论
近年来年轻乳腺癌发病率呈逐年升高趋势,由于这一年龄段面临工作、家庭及诸多因素,所以其治疗方案的选择也需要个体化模式[12-14]。目前局部晚期年轻乳腺癌患者治疗策略为新辅助治疗后综合决策模式,临床中先行新辅助治疗,这样可以降低临床分期,提高乳腺癌手术切除率和乳腺癌保留乳房率及减少手术过程中肿瘤细胞扩散机会,早期杀灭亚临床病灶,同时还可指导术后辅助化疗方案的选择[15-16]。患者经过新辅助治疗后,肿瘤降期,有一部分能达到pCR,然后再行手术,这样不但能提高手术切除率,而且保乳的机会也增加,术后给予放疗、靶向等综合治疗,患者生存时间延长,而且生活质量明显提高[17-19]。
临床中对于HER-2阳性乳腺癌患者新辅助治疗是以曲妥珠单抗为基石的化疗联合靶向治疗方案[20-24],其中化疗药物为紫杉醇,临床上常用的紫杉类药物为溶剂型紫杉醇,在使用中需要聚乙烯蓖麻油作为溶剂,为了预防溶剂带来的过敏反应,患者需要在化疗前使用抗组胺及地塞米松等药物进行预防不良反应,而且输注时间相对较长。白蛋白紫杉醇属于新型紫杉醇制剂,解决的溶剂型紫杉醇诸多缺点,其不含聚氧乙烯蓖麻油,使用时无需进行抗过敏预处理,由于去除了有机溶剂,药物不良反应明显减轻,抗肿瘤活性增高,骨髓抑制减少,使用方法更便利,静脉滴注时间更短[9-11]。CA012研究[25]为白蛋白结合型紫杉醇与溶剂型紫杉醇治疗转移性乳腺癌的头对头III期临床试验,结果显示白蛋白结合型紫杉醇能显著提高客观缓解率(ORR)、延长至疾病进展时间(TTP),3级感觉神经病变缓解时间更短且安全性、耐受性更好。CA024研究[26]为不同剂量的白蛋白结合型紫杉醇一线治疗转移性乳腺癌的II期临床试验,研究结果表明,白蛋白结合型紫杉醇同样显著提高ORR、PFS,4级中性粒细胞减少症发生率明显降低,感觉神经毒性改善时间显著缩短;而且白蛋白结合型紫杉醇在新辅助化疗中可获得更好的cCR,并可一定程度上提高pCR,且不增加治疗所致的不良反应[27]。目前白蛋白结合型紫杉醇在复发转移性乳腺癌患者中广泛应用[27-29],而且国产白蛋白紫杉醇已进入国家4+7采购药品目录,价格相对便宜,患者易接受。因此,笔者对HER-2阳性局部晚期年轻乳腺癌患者应用改良PCH方案,即应用白蛋白紫杉醇替代普通溶剂型紫杉醇,结果令人满意。
本研究结果显示,改良PCH组的cCR、pCR率均明显高于PCH组(43.8% vs.20.0%,χ2=3.997,P=0.046;25.0% vs.6.7%,χ2=4.098,P=0.043)。改良PCH组较PCH组中位无进展生存时间及总生存时间均明显延长(P<0.05)。改良PCH方案较PCH方案比较,能获得更好的cCR,并可一定程度上提高了pCR,显著延长了患者生存时间,这可能与白蛋白结合型紫杉醇的作用机制有关,研究[30]发现白蛋白结合型紫杉醇更有效的调控细胞PI3K/Akt相关通路蛋白的表达水平,影响细胞凋亡相关蛋白水平,促进肿瘤细胞的凋亡进而杀伤肿瘤细胞。不良反应方面,改良PCH组较PCH组比较,粒细胞减少例数明显减少(P<0.05);周围神经毒性方面改良PCH组较高(P<0.05),但主要表现为手足麻木和刺痛,但均未发生3~4度的神经毒性反应。本研究中患者应用白蛋白结合型紫杉醇周围神经病变的发生率可能与累积给药剂量有关,但由于没有助溶剂的影响,周围神经病变缓解更快,患者存在神经毒性症状的时间相对较短。因此,笔者认为改良PCH方案对于HER-2阳性局部晚期年轻乳腺癌患者不失为一种更为优化的治疗方案,更具有个体化治疗理念。
综上所述,改良PCH方案不但提高了患者的cCR率,同时也一定程度上提高了pCR率,患者近期疗效满意,不良反应较轻。2020年CSCO乳腺癌诊治指南[31]对HER-2阳性乳腺癌患者新辅助治疗方案做了更新,TCbPH、THP方案作为I级推荐,对于改良PCH方案的远期疗效,以及与双靶治疗效果的比较,需要后期随访,以作进一步研究。
[1]Fidler MM,Bray F,Soerjomataram I.The global cancer burden and human development:a review[J].Scand J Public Health,2018,46(1):27–36.doi:10.1177/1403494817715400.
[2]Torre LA,Islami F,Siegel RL,et al.Global cancer in women:burden and trends[J].Cancer Epidemiol Biomarkers Prev,2017,26(4):444–457.doi:10.1158/1055–9965.EPI-16–0858.
[3]Moasser MM,Krop IE.The Evolving Landscape of HER2 Targeting in Breast Cancer[J].JAMA Oncol,2015,1(8):1154–1161.doi:10.1001/jamaoncol.2015.2286.
[4]Chen YY,Wang LW,Chen FF,et al.Efficacy,safety and administration timing of trastuzumab in human epidermal growth factor receptor 2 positive breast cancer patients:A metaanalysis[J].Exp Ther Med,2016,11(5):1721–1733.doi:10.3892/etm.2016.3095.
[5]Maximiano S,Magalhães P,Guerreiro MP,et al.Trastuzumab in the Treatment of Breast Cancer[J].BioDrugs,2016,30(2):75–86.doi:10.1007/s40259–016–0162–9.
[6]中国抗癌协会乳腺癌专业委员会.中国抗癌协会乳腺癌诊治指南与规范(2019年版)[J].中国癌症杂志,2019,29(8):609–679.doi:10.19401/j.cnki.1007–3639.2019.08.009.
Chinese Anti-Cancer Association,Committee of Breast Cancer Society.Guidelines and standards for diagnosis and treatment of breast cancer of Chinese Anti-Cancer Association (2019edition)[J].China Oncology,2019,29(8):609–679.doi:10.19401/j.cnki.1007–3639.2019.08.009.
[7]Gradishar WJ,Anderson BO,Balassanian R,et a1.NCCN Guidelines Insights:Breast Cancer,Version 1.2017 [J].J Natl Compr Canc Netw,2017,15(4):433–451.doi:10.6004/jnccn.2017.0044.
[8]江泽飞,邵志敏,徐兵河.人表皮生长因子受体2阳性乳腺癌临床诊疗专家共识2016[J].中华医学杂志,2016,96(14):1091–1096.doi:10.3760/cma.j.issn.0376–2491.2016.14.006.
Jiang ZF,Shao ZM,Xu BH,et al.Expert consensus on clinical diagnosis and treatment of human epidermal growth factor receptor 2 breast cancer 2016[J].National Medical Journal of China,2016,96(14):1091–1096.doi:10.3760/cma.j.issn.0376–2491.2016.14.006.
[9]张惠茹.白蛋白结合型紫杉醇治疗晚期乳腺癌的疗效及安全性[J].中国实用医药,2016,11(23):166.doi:10.14163/j.cnki.11–5547/r.2016.23.118.
Zhang HR.Efficacy and safety of albumin-bound paclitaxel in treatment of advanced breast cancer[J].China Practical Medical,2016,11(23):166.doi:10.14163/j.cnki.11–5547/r.2016.23.118.
[10]Huang H,Shi H,Liu J,et al.Co-delivery of all-trans-retinoic acid enhances the anti-metastasis effect of albumin-bound paclitaxel nanoparticles[J].Chem Commun (Camb),2016,53(1):212–215.doi:10.1039/c6cc08146k.
[11]张岭,王珏,陈锐,等.紫杉醇与白蛋白结合型紫杉醇在HER2阴性乳腺癌新辅助化疗中的疗效研究[J].南京医科大学学报:自然科学版,2018,38(6):807–811.doi:10.7655/NYDXBNS20180618.
Zhang L,Wang J,Chen R,et al.Efficacy observation of paclitaxel and albumin-bound paclitaxel in neoadjuvant chemotherapy for HER2 negative breast cancer[J].Journal of Nanjing Medicial University,2018,38(6):807–811.doi:10.7655/NYDXBNS20180618.
[12]Mi ZH,Ren JS,Zhang HZ,et al.Analysis for the breast cancer screening among urban populations in China,2012–2013[J].Zhong Hua Yu Fang Yi Xue Za Zhi,2016,50(10):887–892.doi:10.3760/cma.j.issn.0253–9624.2016.10.010.
[13]张军,张永庆,王昌亮,等.35岁以下年轻乳腺癌患者的临床特征及预后因素分析[J].现代生物医学进展,2016,16(12):2346–2350.doi:10.13241/j.cnki.pmb.2016.12.039.
Zhang J,Zhang YQ,Wang CL,et al.Clinical Characteristics and Prognostic Factors of Breast Carcinoma in Women Aged 35 Years or Younger[J].Progress in Modern Biomedicine,2016,16(12):2346–2350.doi:10.13241/j.cnki.pmb.2016.12.039.
[14]曹希,徐雅莉,孙强.年龄与三阴性乳腺癌患者预后的关系[J].中国普通外科杂志,2020,29(5):515–524.doi:10.7659/j.issn.1005–6947.2020.05.001.
Cao X,Xu YL,Sun Q.Relationship between age and prognosis in patients with triple-negative breast cancer[J].Chinese Journal of General Surgery,2020,29(5):515–524.doi:10.7659/j.issn.1005–6947.2020.05.001.
[15]Abdel-Razeq H,Saadeh SS,Abu-Nasser M,et al.Four cycles of adriamycin and cyclophosphamide followed by four cycles of docetaxel (NSABP-B27) with concomitant trastuzumab as neoadjuvant therapy for high-risk,early-stage,HER2-positive breast cancer patients[J].Onco Targets Ther,2018,11:2091–2096.doi:10.2147/OTT.S151821.
[16]蔡耿喜,蔡子杰,陈前军,等.乳腺癌新辅助化疗的现状与进展——南方乳腺癌论坛主要议题与共识[J].中国普通外科杂志,2019,28(11):1309–1321.doi:10.7659/j.issn.1005-6947.2019.11.001.
Cai GX,Cai ZJ,Chen QJ,et al.Current status and development of chemotherapy of breast cancer:the main topics and agreements of China South Breast Cancer Symposium[J].Chinese Journal of General Surgery,2019,28(11):1309–1321.doi:10.7659/j.issn.1005–6947.2019.11.001.
[17]Early Breast Cancer Trialists' Collaborative Group (EBCTCG).Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer:meta-analysis of individual patient data from ten randomized trials[J].Lancet Oncol,2018,19(1):27–39.doi:10.1016/S1470–2045(17)30777–5.
[18]朱月梅,王国如,张沂,等.表柔比星联合紫杉醇新辅助化疗治疗乳腺癌保乳术患者的疗效及安全性[J].中国普通外科杂志,2019,28(11):1406–1413.doi:10.7659/j.issn.1005–6947.2019.11.014.
Zhu YM,Wang GR,Zhang Y,et al.Efficacy and safety of neoadjuvant chemotherapy with epirubicin plus paclitaxel in treatment of patients undergoing breast-preserving surgery for breast cancer[J].Chinese Journal of General Surgery,2019,28(11):1406–1413.doi:10.7659/ j.issn.1005–6947.2019.11.014.
[19]Cortazar P,Zhang L,Untch M,et al.Pathological complete response and long-term clinical benefit in breast cancer:the CTNeoBC pooled analysis[J].Lancet,2014,384(9938):164–172.doi:10.1016/S0140–6736(13)62422–8.
[20]Schettini F,Buono G,Cardalesi C,et al.Hormone receptor/human epidermal growth factor receptor 2 positive breast cancer:Where we are now and where we are going?[J].Cancer Treat Rev,2016,46:20–26.doi:10.1016/j.ctrv.2016.03.012.
[21]宋敏,赵静,陈丽艳,等.曲妥珠单抗联合多西紫杉醇在HER-2阳性晚期乳腺癌中的应用[J].现代生物医学进展,2017,17(6):1152–1155.doi:10.13241/j.cnki.pmb.2017.06.040.
Song M,Zhao J,Chen LY,et al.Application of Trastuzumab combined Docetaxel in Treatment of HER-2 Positive Advanced Breast Cancer[J].Progress in Modern Biomedicine,2017,17(6):1152–1155.doi:10.13241/j.cnki.pmb.2017.06.040.
[22]孙丽妮.赫赛汀在转移性乳腺癌治疗中的应用探讨[J].中华肿瘤防治杂志,2018,25(S2):36–37.doi:10.16073/j.cnki.cjcpt.2018.s2.023.
Sun LN.Application of herceptin in treatment of metastatic breast cancer[J].Chinese Journal of Cancer Prevention and Treatment,2018,25(S2):36–37.doi:10.16073/j.cnki.cjcpt.2018.s2.023.
[23]Pondé N,Brandão M,El-Hachem G,et al.Treatment of advanced HER2-positive breast cancer:2018 and beyond[J].Cancer Tteat Rev,2018,67:10–20.doi:10.1016/j.ctrv.2018.04.016.
[24]何敏,邓丽聪.赫赛汀联合紫杉醇新辅助化疗对HER-2阳性乳腺癌细胞增殖活力的影响[J].海南医学院学报,2018,24(17):1603–1606.doi:10.13210/j.cnki.jhmu.20180829.005.
He M,Deng LC.Effect of Herceptin combined with paclitaxel neoadjuvant chemotherapy on proliferation via-bility of HER-2 positive breast cancer cell[J].Journal of Hainan Medical University,2018,24(17):1603–1606.doi:10.13210/j.cnki.jhmu.20180829.005.
[25]Gradishar WJ,Tjulandin S,Davidson N,et al.Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer[J].J Clin Oncol,2005,23(31):7794–7803.doi:10.1200/JCO.2005.04.937.
[26]Gradishar WJ,Krasnojon D,Cheporov S,et al.Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer[J].J Clin Oncol,2009,27(22):3611–3619.doi:10.1200/JCO.2008.18.5397.
[27]Untch M,Jackisch C,Schneeweiss A,et al.Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69):a randomised,phase 3 trial[J].Lancet Oncol,2016,17(3):345–356.doi:10.1016/S1470–2045(15)00542–2.
[28]Futamura M,Nagao Y,Ishihara K,et al.Preoperative neoadjuvant chemotherapy using nanoparticle albumin-bound paclitaxel followed by epirubicin and cyclophosphamide for operable breast cancer:a multicenter phase II trial[J].Breast Cancer,2017,24(4):615–623.doi:10.1007/s12282–016–0748–6.
[29]Gluz D,Nitz U,Liedtke C,et al.Impact of 12 weeks nabpaclitaxel+carboplatin or gemcitabine followed by anthracycline administration according to pCR in triple-negative early breast cancer:Survival results of WSG-ADAPT-TN phase II trial[J].J Clin Oncol,2018,36(15 suppl):573.doi:10.1200/JCO.2018.36.15_suppl.573.
[30]余峰,刘晶晶,张晟,等.白蛋白紫杉醇用于乳腺癌新辅助化疗的二期临床研究[J].中华乳腺病杂志:电子版,2014,8(4):9–15.doi:10.3877/cma.j.issn.1674–0807.2014.04.003.
A phase II clinical study of albumin-bound paclitaxel used in neoadjuvant therapy in breast cancer women[J].Chinese Journal of Breast Disease:Electronic Version,2014,8(4):9–15.doi:10.3877/cma.j.issn.1674–0807.2014.04.003.
[31]中国临床肿瘤学会指南工作委员会.中国临床肿瘤学会(CSCO)乳腺癌诊疗指南2020[M].北京:人民卫生出版社,2020:1–148.
Guidelines Working Committee of Chinese Society of Clinical Oncology.Guidelines for diagnosis and treatment of breast cancer of the Chinese Society of Clinical Oncology (CSCO) 2020[M].Beijing:People's Medical Publishing House,2020:1–148.
