主动脉分为内膜(基底膜上的内皮细胞)、中膜(弹性蛋白和平滑肌细胞)、外膜(胶原纤维和成纤维细胞)3 层,动态变化着的细胞和细胞外基质通过复杂的相互作用来响应心脏射血所产生的高强度血流动力学变化,其中任何一环出现问题都有可能导致主动脉夹层(aortic dissection,AD),其中以胸主动脉夹层(thoracic aorta dissection,TAD)最为多见。TAD因涉及范围不同被分为Stanford A型(涉及升主动脉)和Stanford B型(未涉及升主动脉),其主要病理特征为平滑肌细胞凋亡、细胞外基质降解、炎症细胞浸润、蛋白多糖蓄积[1-2]。对于AD的研究每种模型都有其擅长的领域,啮齿动物基因组研究广泛,生命周期短,常用化学诱导或转基因方式建模,对AD发病机制的研究最有优势。基因模型主要以基因敲除、基因过表达、或病毒转染等方式来研究单个或多个基因对AD的影响。化学诱导模型是通过药物或饮食调整的方式诱导动物产生AD。虽然现在也有通过主动脉显微手术诱导的小动物模型[3],但由于体量太小在手术改进、器械测试和血流动力学评估等方面与大动物没有可比性。大动物模型的局限性在于无法复制AD的内侧变性,目前主要是通过外科手术或腔内途径侵入性建模,以求外观上和夹层达到统一,病理生理方面更接近创伤性或医源性AD。
1 小动物模型
1.1 化学或饮食诱导的动物模型
β-氨基丙腈(β-a m i n o p r o p i o n i t r i l e,BAPN)是赖氨酰氧化酶抑制剂,可破坏细胞外基质微环境[4] 。Ang II除了能升高血压还有其他非血流动力学作用[5]。Ang II与BAPN联合使用是目前最常用的化学诱导模型。Kurihara等[6]首次用BAPN/Ang II联合诱导的方式诱发AD。他们先用BAPN预处理小鼠,再给Ang II或NE。结果显示,BAPN/Ang II组在泵入Ang II 6 h后开始产生AD,24 h 100%产生AD,而BAPN/NE组或BAPN组成功率仅10%。后来Ren等[7]用相同方法使C57BL/6和FVB两种遗传背景的小鼠也全部产生了AD,这说明此模型可复制性极高。BAPN通过破坏未成熟小鼠主动脉壁的完整性,诱使主动脉内侧变性,再经Ang II处理后,慢性主动脉瘤转为AD。近期Izawa-Ishizawa等[8]在用BAPN/Ang II前先用亚硝基左旋精氨酸甲酯(Nω-nitro-L-arginine methyl ester,L-NAME)预处理小鼠,L-NAME是NO合酶抑制剂,可使内皮氧化应激而加重AD。结果发现L-NAME组AD患病率显著提升,内侧变性也更明显。虽然之前已经有了成功率1 0 0%的化学诱导模型,但该模型引入内皮损伤因素验证了匹伐他汀能通过抑制内皮氧化应激预防AD的产生。最近Nishida等[9]在Ang II/BAPN的基础上加盐水诱导,致AD破裂率增加,发现高盐对AD的恶化作用与IL-17有很大关系。此外,还有用高脂饮食和Ang II联合构建的散发性AD模型,LeMaire等[10]用该模型验证了环丙沙星对AD的恶化作用。以上小鼠化学或饮食诱导模型具体操作见图1。
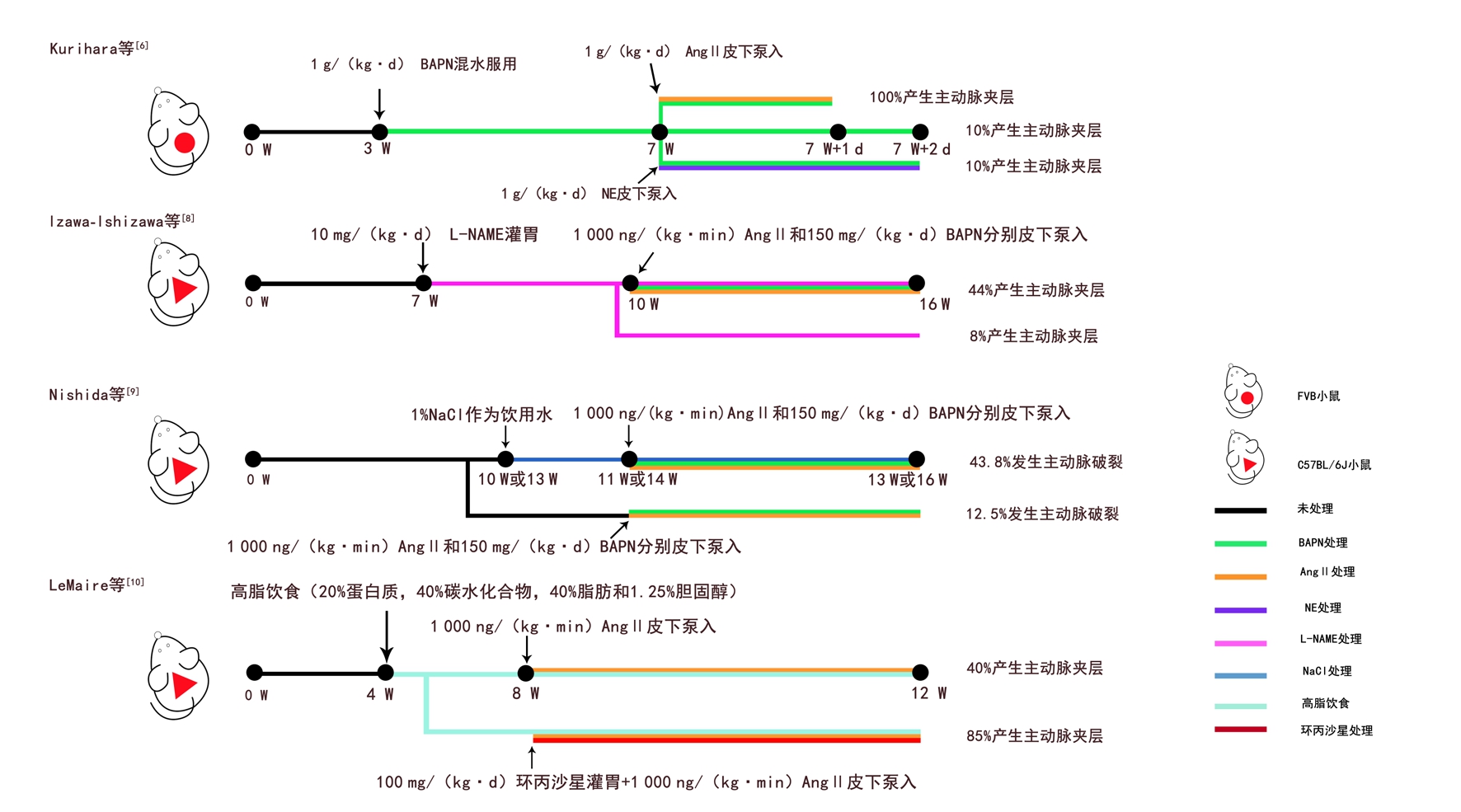
图1 小鼠化学或饮食诱导模型
Figure 1 Chemical- or diet-induced mice models
1.2 基因动物模型
转基因动物模型主要用于遗传性TAD的研究,遗传性TAD主要有Marfan综合征、Loeys-Dietz综合征、血管性Ehlers-Danlos综合征3种。Marfan综合征小鼠主要有F b n 1mgΔ/mgΔ、F b n 1mgR/mgR、Fbn1C1039G/+ 3种,Fbn1 mgΔ/mgΔ、Fbn1mgR/mgR小鼠分别在出生后3周、1年内死亡[11-12],Fbn1C1039G/+杂合小鼠不会死于AD,2个月后出现内侧变性[13],常与化学诱导联用[14]。LDS小鼠主要有Tgfbr1M318R/+、Tgfbr2G357W/+、Tgfb1+/-、Tgfb2+/-、Smad3+/-,其中单倍体不足小鼠(Tgfb1 +/-、Tgfb2+/-)单个等位基因的功能丧失就足以引起主动脉根动脉瘤,但杂合错义突变小鼠(Tgfbr1M318R/+、Tgfbr2G357W/+)却更能代表Loeys-Dietz 综合征的血管表型[15-18]。血管性Ehlers-Danlos综合征小鼠中只有+/Col3a1△、Col3a1G209S/+、Col3a1G938D/+小鼠能产生少量AD[19-20]。
2 大动物模型
2.1 通过外科手术创建的动物模型
2.1.1 Stanford B 型主动脉夹层(type B aortic dissection,TBAD)大动物模型 首个TBAD 大动物模型是1959年Blanton 等[21]通过外科手术创建的,后来很多研究对此进行了优化,但基本操作变化不大:选犬或猪,左侧开胸,截断血流,环形切开主动脉外膜,在内膜和外膜间用各种方法制造AD,切开内膜,缝合外膜。Fujii 等[22] 在假腔产生后用手捏搓使其扩大延展,并在切开内膜后,采用上全下外(裂口上方全层缝合,裂口下方仅缝合外膜和部分中膜)的缝合方式维持假腔通畅。结果显示用此方法的3 头猪都有较为可观的成活率和假腔通畅率,他们的另一组实验还提示猪对戊巴比妥麻醉并不耐受。Terai 等[23] 创建了首个有稳定继发破口的TBAD 大动物模型:将夹层小范围分离出来后,用生理盐水延长假腔,后用8 F 橡胶管固定假腔形状,在距第一破口4 cm 处造第二破口,夹层范围也止于此,缝合方式类似Fujii 等[22]。用此法建模,夹层范围固定,两年通畅率极高,但经长期观察并无内侧变性和血栓发生。Cui 等[24]提出了一种新的内膜固定方法:他们将内膜瓣两侧分别折叠缝合在主动脉侧壁上,用这种内膜双角缝合的方式维持夹层破口的稳定开放,成功率91%。Tang 等[25] 创建了两种模型:不可控模型是在Cui 等[24] 的基础上再升高血压,可控模则类似Terai 等[25]。实验发现NE 用量若控制不当会使假腔迅速扩张导致动物死亡率升高。之后Wang 等[26] 在假腔延伸到膈以上时就用硝酸甘油阻断其扩张趋势,增加了AD 的可控性。最近Guo 等[27] 用10 头上海长白猪成功建模:鼻夹剥离中膜,上全下外缝合内膜,再升高血压,成功率100%。最长存活时间2年,最长波及范围147.6 mm。通过该实验发现大部分继发破口位于分支动脉开口处,并在此处中止,而且内膜瓣撕裂位置越靠近外膜,波及范围越广。
2.1.2 Stanford A 型动脉夹层(type A aortic dissection,TAAD)大动物模型 由于病情凶险,病死率高,关于TAAD 的慢性大动物模型几乎没有。Papper 等[28] 于1970年同时创建了TAAD 和TBAD 犬模型,基本步骤同上,不过分离中膜采用的是CO2,结果发现TAAD 组5 d 内病死率高达87.5%,TBAD 组用相同方法却造夹乏力,仅形成很有限的假腔,说明创建TAAD 模型时并不能完全照搬TBAD 的建模方法,还需结合自身血流动力学特征和组织学特性。Li 等[29] 在升主动脉起始远端2 cm 处切开外膜,再用文氏钳钝性分离1 cm,用生理盐水扩大假腔后用弹性蛋白酶灌注5 min,最后内膜双角缝合,升高血压,发现AD最远可达肾动脉开口处,动物产生全身炎症反应、多器官功能受损。Qin 等[30] 后来也用类似方法制造了TAAD。
2.2 通过腔内手术创建的动物模型
2.2.1 TBAD 大动物模型 1998年Razavi 等[31]经腔内创建了首个TBAD 大动物模型。他们通过颈总动脉或股动脉两条入路分别顺行或逆行制造夹层,由于方法类似,这里以颈总动脉为例:肝穿针由9 F 血管鞘进入,在距左锁骨下动脉开口远端4 cm 处,将针尖刺入主动脉壁后注射造影剂分离中膜,后将导丝引入假腔,弯曲成环,与造影剂一同将假腔延伸至腹主动脉,最后用真腔内的肝穿针刺破假腔内的球囊产生继发破口,再用球囊扩张破口。该模型成功率约75%,6 只出现真腔狭窄,但无随访记录。Eggebrecht 等[32] 将此模型成功用于MRI 下支架植入术的测试。后来Okuno 等[33]改良了此法,他们将改良10 F 外导管(图2)在肾动脉下2 cm 处刺入动脉壁,后用导丝和4 F 直导管从下往上逆行剥离中膜。该模型在器械上进行了创新,成功率约78.6%。术中使用的改良导管可以更好的估计进针深度。El Batti 等[34] 则是通过Outback 导管重返真腔产生近端破口的,成功率82%,但此模型真腔压力大于假腔,与临床事实不符。
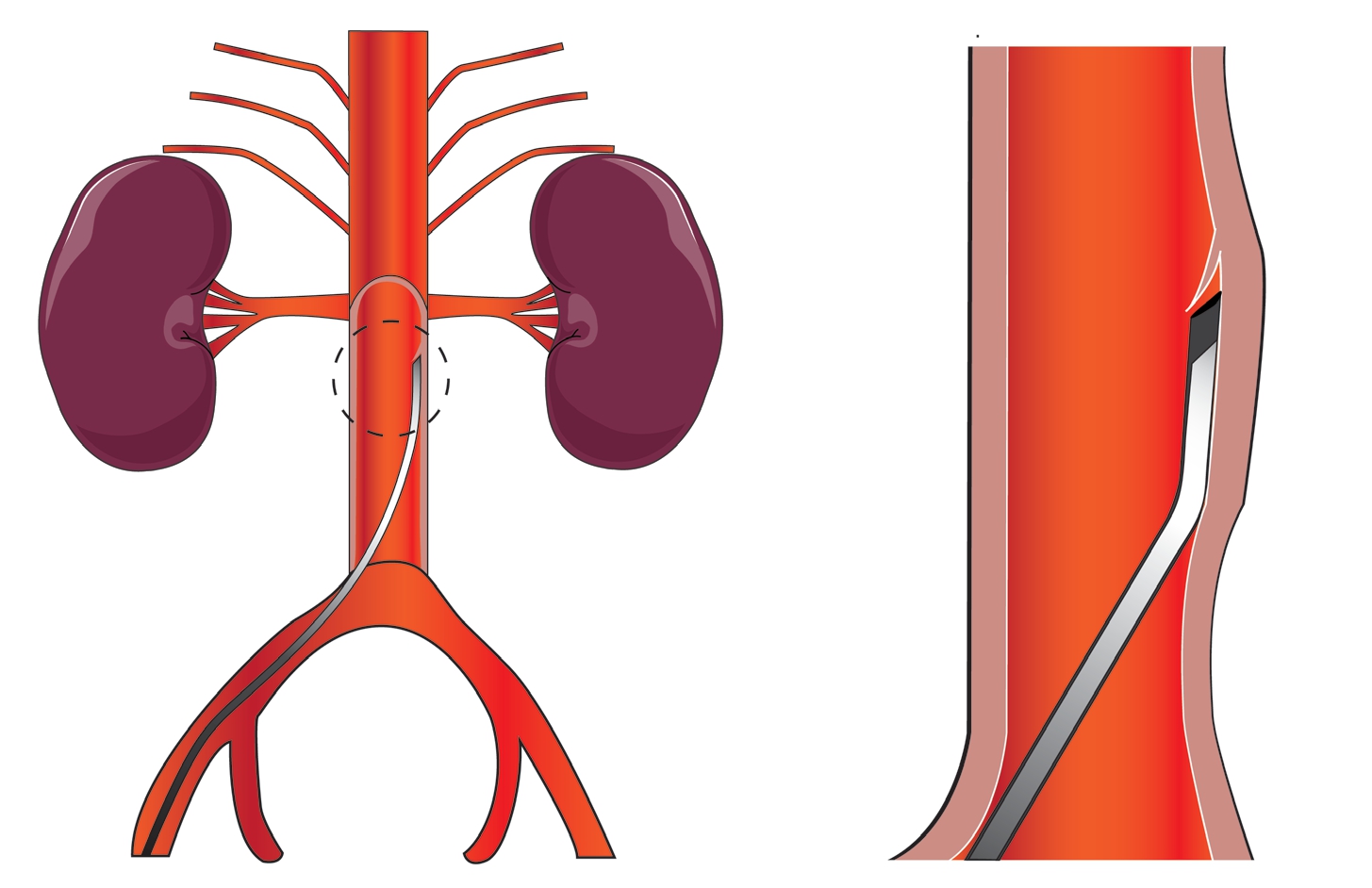
图2 外导管操作示意图(外导管尖端被削成适应主动脉壁的角度,刺破主动脉内膜,创建初始破口)
Figure 2 Schematic representation of the operation of the outer catheter(the tip of the outer catheter cut in an angle adapted to the aortic wall,and puncturing the aortic intima to create an initial rupture)
2.2.2 TAAD 大动物模型 2018年Boufi 等[35]经腔内创建了TAAD 大动物模型,自心尖插入8 F 改良鞘管,调整好角度,再用Outback 导管(A 组)或EchoTip Ultra 内镜超声针(B 组)刺破主动脉壁并向前推进,随后用造影剂和成环导丝一起顺行延伸假腔,之后将导丝环缩至锋利刺破内膜瓣形成继发破口并有球囊扩张。结果显示A 组产生的假腔范围有限,平均长度仅23 mm,无继发破口,成功率66%。B 组假腔平均长度110 mm,7 只产生继发破口,成功率80%,此外B 组还能观察到AD 长度与继发破口的位置有一定关系,这在以往的血流动力学研究中也有说明[36],或许以后可以通过调整继发破口位置来控制AD。从该实验可以看出经心尖入路能更便捷的进入升主动脉,且实验中所用的超声针可标记入针深度,腔内建模的技术难度正随着新器材的出现而不断下降。腔内模型不破坏主动脉正常结构,更符合夹层病理特点,在术式和器械评估方面略胜于外科模型。最近就有研究用腔内模型评估了一种新型开窗剪的实用性[34]。
3 总结
小动物目前已经产生了成功率100%的化学诱导模型,而且还能通过对不同基因和化学诱导剂的灵活调节来研究各种分子机制。大动物模型操作难度较高,可复制性不如小动物模型。就手术治疗来说,TBAD具有更稳定的大动物模型,其腔内发展也更迅速[2,37-39]。与此相比,虽然已经诞生了首个FDA批准的专用于TAAD的腔内器材[40],但目前较多TAAD新技术仍只能通过离体模型[41]、健康大动物[42]进行临床前评估,得出的结果难免刻板。大动物模型的局限在很大程度上制约了TAAD的手术进步。目前外科手术仍然是TAAD的推荐术式[43]。TAAD的腔内治疗仍然存在瓶颈和困难,这都需要更加完善的动物模型来进行实践。对这些临床难题的模拟也会是未来大动物模型的一个发展趋势,或许可以尝试用Ang II、BAPN或高脂饮食来辅助建模。
[1]Shen YH,LeMaire SA,Webb NR,et al.Aortic Aneurysms and Dissections Series[J].Arterioscler Thromb Vasc Biol,2020,40(3):e37-46.doi:10.1161/ATVBAHA.120.313991.
[2]Evangelista A,Isselbacher E M,Bossone E,et al.Insights From the International Registry of Acute Aortic Dissection:A 20-Year Experience of Collaborative Clinical Research[J].Circulation,2018,137(17):1846-1860.doi:10.1161/CIRCULATIONAHA.117.031264.
[3]Matt P,Huso DL,Habashi J,et al.Murine model of surgically induced acute aortic dissection type A[J].J Thorac Cardiovasc Surg,2010,139(4):1041-1047.doi:10.1016/j.jtcvs.2009.08.039.
[4]Xia L,Sun C,Zhu H,et al.Melatonin protects against thoracic aortic aneurysm and dissection through SIRT1-dependent regulation of oxidative stress and vascular smooth muscle cell loss[J].J Pineal Res,2020:e12661.doi:10.1111/jpi.12661.
[5]Forrester SJ,Booz GW,Sigmund CD,et al.Angiotensin II Signal Transduction:An Update on Mechanisms of Physiology and Pathophysiology[J].Physiol Rev,2018,98(3):1627-1738.doi:10.1152/physrev.00038.2017.
[6]Kurihara T,Shimizu-Hirota R,Shimoda M,et al.Neutrophilderived matrix metalloproteinase 9 triggers acute aortic dissection[J].Circulation,2012,126(25):3070-3080.doi:10.1161/CIRCULATIONAHA.112.097097.
[7]Ren W,Liu Y,Wang X,et al.beta-Aminopropionitrile monofumarate induces thoracic aortic dissection in C57BL/6 mice[J].Sci Rep,2016,6:28149.doi:10.1038/srep28149.
[8]Izawa-Ishizawa Y,Imanishi M,Zamami Y,et al.Development of a novel aortic dissection mouse model and evaluation of drug efficacy using in-vivo assays and database analyses[J].J Hypertens,2019,37(1):73-83.doi:10.1097/HJH.0000000000001898.
[9]Nishida N,Aoki H,Ohno-Urabe S,et al.High Salt Intake Worsens Aortic Dissection in Mice:Involvement of IL(Interleukin)-17A-Dependent ECM(Extracellular Matrix)Metabolism[J].Arterioscler Thromb Vasc Biol,2020,40(1):189-205.doi:10.1161/ATVBAHA.119.313336.
[10]LeMaire S A,Zhang L,Luo W,et al.Effect of Ciprofloxacin on Susceptibility to Aortic Dissection and Rupture in Mice[J].JAMA Surg,2018,153(9):e181804.doi:10.1001/jamasurg.2018.1804.
[11]Pereira L,Andrikopoulos K,Tian J,et al.Targetting of the gene encoding fibrillin-1 recapitulates the vascular aspect of Marfan syndrome[J].Nat Genet,1997,17(2):218-222.doi:10.1038/ng1097-218.
[12]Pereira L,Lee SY,Gayraud B,et al.Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1[J].Proc Natl Acad Sci U S A,1999,96(7):3819-3823.doi:10.1073/pnas.96.7.3819.
[13]Judge DP,Biery NJ,Keene DR,et al.Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome[J].J Clin Invest,2004,114(2):172-181.doi:10.1172/JCI20641.
[14]Oller J,Méndez-Barbero N,Ruiz E J,et al.Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome[J].Nat Med,2017,23(2):200-212.doi:10.1038/nm.4266.
[15]MacFarlane EG,Parker SJ,Shin JY,et al.Lineage-specific events underlie aortic root aneurysm pathogenesis in Loeys-Dietz syndrome[J].J Clin Invest,2019,129(2):659-675.doi:10.1172/JCI123547.
[16]Lindsay ME,Schepers D,Bolar NA,et al.Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm[J].Nat Genet,2012,44(8):922-927.doi:10.1038/ng.2349.
[17]Gallo EM,Loch DC,Habashi JP,et al.Angiotensin II-dependent TGF-beta signaling contributes to Loeys-Dietz syndrome vascular pathogenesis[J].J Clin Invest,2014,124(1):448-460.doi:10.1172/JCI69666.
[18]Huang X,Yue Z,Wu J,et al.MicroRNA-21 Knockout Exacerbates Angiotensin II-Induced Thoracic Aortic Aneurysm and Dissection in Mice With Abnormal Transforming Growth Factor-beta-SMAD3 Signaling[J].Arterioscler Thromb Vasc Biol,2018,38(5):1086-1101.doi:10.1161/ATVBAHA.117.310694.
[19]Smith LB,Hadoke PW,Dyer E,et al.Haploinsufficiency of the murine Col3a1 locus causes aortic dissection:a novel model of the vascular type of Ehlers-Danlos syndrome[J].Cardiovasc Res,2011,90(1):182-190.doi:10.1093/cvr/cvq356.
[20]Bowen CJ,Calderón Giadrosic JF,Burger Z,et al.Targetable cellular signaling events mediate vascular pathology in vascular Ehlers-Danlos syndrome[J].J Clin Invest,2020,130(2):686-698.doi:10.1172/JCI130730.
[21]Blanton FS Jr,Muller WH Jr,Warren WD.Experimental production of dissecting aneurysms of the aorta[J].Surgery,1959,45(1):81-90.
[22]Fujii H,Tanigawa N,Okuda Y,et al.Creation of aortic dissection model in swine[J].Jpn Circ J,2000,64(9):736-737.doi:10.1253/jcj.64.736.
[23]Terai H,Tamura N,Yuasa S,et al.An experimental model of Stanford type B aortic dissection[J].J Vasc Interv Radiol,2005,16(4):515-519.doi:10.1097/01.RVI.0000151142.80319.85.
[24]Cui JS,Zhuang SJ,Zhang J,et al.Two-end intimal flap suturing method for establishing Stanford B type aortic dissection in a canine model[J].Eur J Vasc Endovasc Surg,2009,38(5):603-607.doi:10.1016/j.ejvs.2009.07.005.
[25]Tang J,Wang Y,Hang W,et al.Controllable and uncontrollable Stanford type B aortic dissection in canine models[J].Eur Surg Res,2010,44(3/4):179-184.doi:10.1159/000283218.
[26]Wang LX,Wang YQ,Guo DQ,et al.An experimental model of Stanford type B aortic dissection with intravenous epinephrine injection[J].Kaohsiung J Med Sci,2013,29(4):194-199.doi:10.1016/j.kjms.2012.08.033.
[27]Guo B,Dong Z,Pirola S,et al.Dissection Level Within Aortic Wall Layers is Associated with Propagation of Type B Aortic Dissection:A Swine Model Study[J].Eur J Vasc Endovasc Surg,2019,58(3):415-425.doi:10.1016/j.ejvs.2019.02.026.
[28]Pappas G,Burquist J.Creation of dissecting thoracic aortic aneurysms in dogs[J].J Surg Res,1970,10(7):333-336.doi:10.1016/0022-4804(70)90052-1.
[29]Li M,Luo N,Bai Z,et al.A canine model of multiple organ dysfunction following acute type-A aortic dissection[J].Surg Today,2012,42(9):876-883.doi:10.1007/s00595-011-0073-9.
[30]Qin C,Zhang H,Gu J,et al.Dynamic monitoring of platelet activation and its role in post-dissection inflammation in a canine model of acute type A aortic dissection[J].J Cardiothorac Surg,2016,11(1):86.doi:10.1186/s13019-016-0472-5.
[31]Razavi MK,Nishimura E,Slonim S,et al.Percutaneous creation of acute type-B aortic dissection:an experimental model for endoluminal therapy[J].J Vasc Interv Radiol,1998,9(4):626-632.doi:10.1016/s1051-0443(98)70333-1.
[32]Eggebrecht H,Kühl H,Kaiser GM,et al.Feasibility of real-time magnetic resonance-guided stent-graft placement in a swine model of descending aortic dissection[J].Eur Heart J,2006,27(5):613-620.doi:10.1093/eurheartj/ehi732.
[33]Okuno T,Yamaguchi M,Okada T,et al.Endovascular creation of aortic dissection in a swine model with technical considerations[J].J Vasc Surg,2012,55(5):1410-1418.doi:10.1016/j.jvs.2011.10.088.
[34]El Batti S,Ben Abdallah I,Dufetelle E,et al.Experimental Evaluation of Endovascular Fenestration Scissors in an Ovine Model of Aortic Dissection[J].Eur J Vasc Endovasc Surg,2018,56(3):373-380.doi:10.1016/j.ejvs.2018.05.024.
[35]Boufi M,Claudel M,Dona B,et al.Endovascular creation and validation of acute in vivo animal model for type A aortic dissection[J].J Surg Res,2018,225:21-28.doi:10.1016/j.jss.2017.12.015.
[36]Canchi S,Guo X,Phillips M,et al.Role of Re-entry Tears on the Dynamics of Type B Dissection Flap[J].Ann Biomed Eng,2018,46(1):186-196.doi:10.1007/s10439-017-1940-3.
[37]Guo Y,Cai H,Yang B,et al.Simultaneous Endovascular Repair for Thoracic and Abdominal Aortic Pathologies:Early and Midterm Results[J].Ann Vasc Surg,2017,40:178-182.doi:10.1016/j.avsg.2016.08.019.
[38]罗明尧,朱梦依,方坤,等.合并严重冠心病的短锚 定区主动脉弓降部疾病的腔内修复[J].中国普通外科杂志,2017,26(12):1536-1540.doi:10.3978/j.issn.1005-6947.2017.12.005.
Luo MY,Zhu MY,Fang K,et al.Endovascular repair of proximal descending aortic disease with insufficient proximal landing zone in patients with concomitant severe coronary heart disease[J].Chinese Journal of General Surgery,2017,26(12):1536-1540.doi:10.3978/j.issn.1005-6947.2017.12.005.
[39]王沫,舒畅,张惟常,等.Stanford B型主动脉夹层合 并迷走右锁骨下动脉的腔内治疗:附1 6 例报告[J].中国普通外科 杂志,2020,29(10):1234-1242.doi:10.7659/j.issn.1005-6947.2020.10.010.
Wang M,Shu C,Zhang WC,et al.Endovascular therapy of Stanford type B aortic dissection combined with aberrant right subclavian artery:a report of 16 cases[J].Chinese Journal of General Surgery,2020,29(10):1234-1242.doi:10.7659/j.issn.1005-6947.2020.10.010.
[40]Muehle A,Chou D,Shah A,et al.Experiences with ascending aortic endografts using FDA IDE approved devices.What has been done,what lesions can be treated and what challenges remain[J].J Cardiovasc Surg(Torino),2015,56(1):11-18.
[41]Gandet T,Seghrouchni A,Ozdemir BA,et al.Experimental evaluation of homemade distal stent graft fenestration for thoracic endovascular aortic repair of type A dissection by a transapical approach[J].J Vasc Surg,2018,68(4):1217-1224.doi:10.1016/j.jvs.2017.08.095.
[42]Qin J,Zhao Z,Wang R,et al.In Situ Laser Fenestration Is a Feasible Method for Revascularization of Aortic Arch During Thoracic Endovascular Aortic Repair[J].J Am Heart Assoc,2017,6(4):e004542.doi:10.1161/JAHA.116.004542.
[43]Tsilimparis N,Drewitz S,Detter C,et al.Endovascular Repair of Ascending Aortic Pathologies with Tubular Endografts:A Single-Center Experience[J].J Endovasc Ther,2019,26(4):439-445.doi:10.1177/1526602819852083.
