胰腺根治性切除术加区域淋巴结清扫是目前胰腺癌的主要治疗方法。胰腺癌患者在根治性切除后的5年生存率为20.7~23.9%,中位生存期22.3~23.6个月[1-3]。日本JASPAC-01方案中报道称将S-1作为术后辅助化疗,可使I~III期胰腺癌患者的5年总生存率提高到44%[4]。受经济条件、医疗水平的影响,而我国胰腺癌患者5年总生存率不足10%[5]。因此,监测胰腺癌术后具有高复发风险的患者,及时实施临床干预,从而改善此类患者的预后具有重要临床价值。糖类抗原19-9(CA19-9)是目前国际指南推荐的临床监测指标[6],但大约有20%的胰腺癌患者术前血清CA19-9水平是正常的[7]。因此,需要寻找兼具敏感性和特异性的指标对胰腺癌患者进行术前评估。
白蛋白-胆红素(albumin-bilirubin,ALBI)评分最初用于评估肝癌患者的肝脏功能障碍的严重程度[8-10],相关研究结果认为ALBI评分在预测肝癌患者的长期生存方面比Child-Pugh评分准确性更佳[8-10]。同时,计算ALBI评分只需要测量血清胆红素浓度和白蛋白水平,相较于Child-Pugh评分可操作性和客观性更强。因此,ALBI评分作为预后指标更容易被广泛推广。虽然现阶段关于ALBI评分的研究大多都集中在肝癌领域,但已有关于ALBI评分预测胃癌患者的预后的报道[1 1-1 2],这表明ALBI可能成为其他癌症的一种有用的预后评估指标。据文献所知,目前还没有研究显示ALBI评分在评估胰腺癌患者预后方面的有效性。因此,本研究探讨ALBI评分对胰腺癌患者的预后意义,以及ALBI评分与血清CA19-9联合应用的临床意义。
1 资料与方法
1.1 一般资料
本研究回顾性分析了2010年1月—2014年11月在辽宁省健康产业集团阜新矿总医院普通外科行胰腺癌根治性切除术的97例患者的临床资料,其中男70例,女27例。患者或患者家属知晓全部诊疗计划,并签署知情同意书。
纳入标准:⑴ 经术后病理学检查确诊为胰腺癌者;⑵ 术前影像学检查明确无远处转移者;⑶ 具有完整的临床病理资料。排除标准:⑴ 术前合并其它脏器存在严重基础疾病,无法耐受手术者;⑵ 术后病理学检查切缘阳性者;⑶ 临床病理资料缺失者;⑷ 术前存在远处转移或术中发现存在腹膜转移者。
1.2 观察指标
全部患者抽取手术当日清晨静脉血,检测血常规、肝肾功及肿瘤学标记物等,以获取血清白蛋白水平、总胆红素浓度和CA19-9水平。ALBI分数计算如下[1 4]:[-0.0852×血清白蛋白水平(g/L)]+[0.66×log总胆红素浓度(μmol/L)];ALBI≤-2.6为1级、-2.6~≤-1.39为2级、>-1.39为3级。
根据《美国癌症联合委员会(AJCC)胰腺癌分期手册》第8版[13]对切除标本的病理特征进行分类。患者接受胰十二指肠切除术,胰体尾切除术和全胰切除术伴区域淋巴结清扫。对入组患者全部进行术后随访,术后3 年内每3 个月复查血清学指标,3年以上每6个月复查血清学指标,每6个月进行1次胸部和腹部CT扫描。总生存期被定义为从手术之日到因任何原因死亡之日或随访截止日,随访截止到2019年11月30日。
1.3 统计学处理
应用SPSS 17.0统计软件进行分析,采用χ2检验评价患者ALBI评分的临床病理特征差异,采用Spearman秩相关系数分析血清白蛋白水平和胆红素浓度之间的相关性,依据Cox比例危险模型对考虑影响总生存预后的因素进行单因素和多因素分析,P<0.05为差异有统计学意义。
2 结 果
2.1 血清白蛋白水平与胆红素浓度相关性
97 例胰腺癌患者的血清白蛋白水平和胆红素浓度中位数分别为39.4(21.1 ~49.5)g/L 和213.9(104.2~406.2)μmol/L,相关性分析结果显示,血清白蛋白水平与胆红素浓度呈明显相关(r=0.45,P=0.018)(图1)。
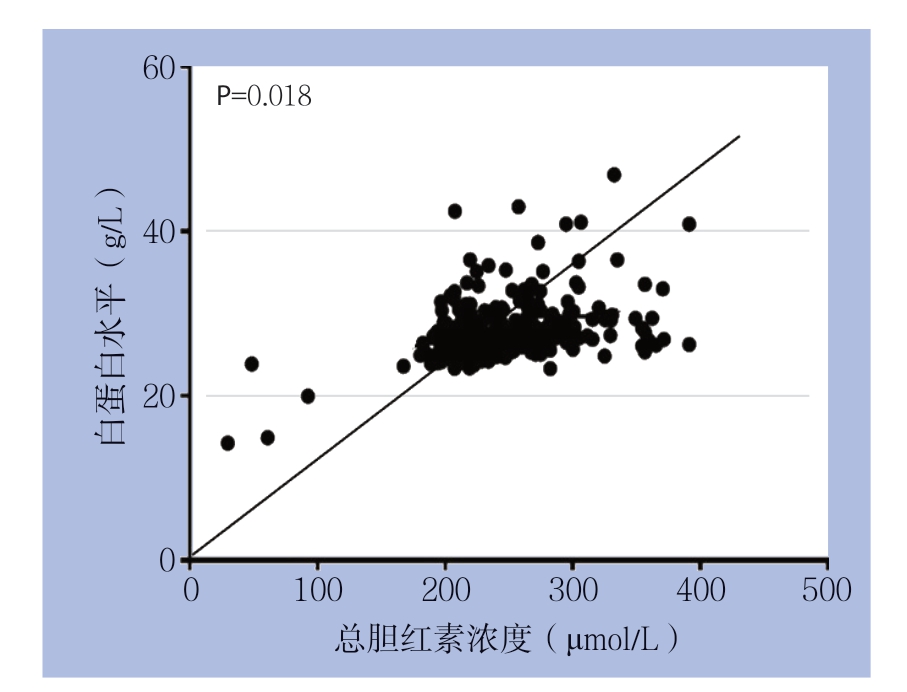
图1 胰腺癌患者总胆红素浓度与白蛋白水平之间的相关性
Figure 1 Correlation between total bilirubin concentration and albumin level in patients with pancreatic cancer
2.2 ALBI 评分与患者临床病理特征的关系
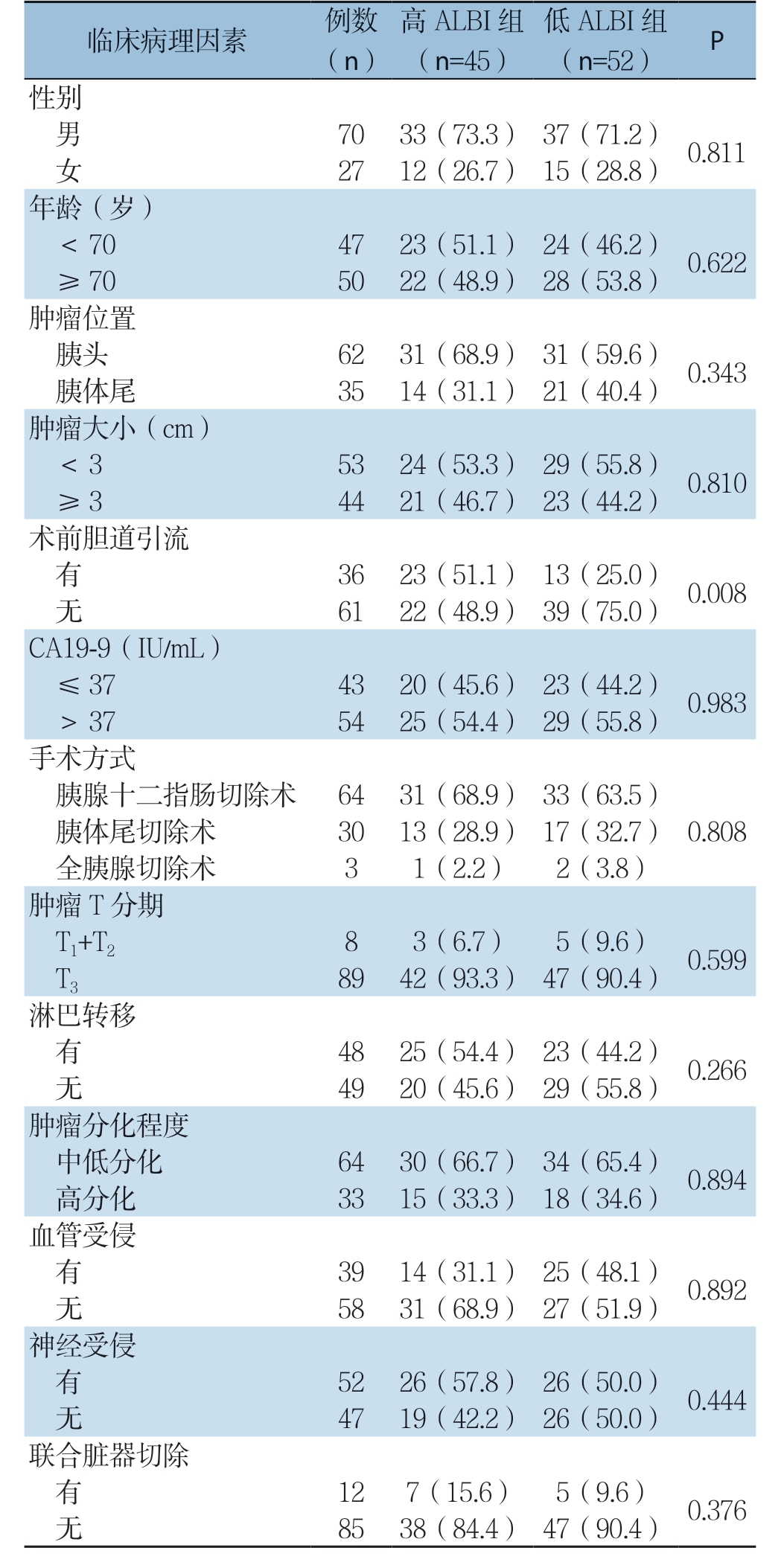
97例胰腺癌患者的ALBI评分中位数为-2.59(-3.63~-1.18)。ALBI 1、2、3级患者分别为52例、43例、2例。鉴于仅有2例患者为ALBI 3级,故将患者分为低ALBI组(ALBI 1级)和高ALBI组(ALBI 2级和3级),对两组各项临床病理特征进一步分析。结果显示,高ALBI组和低ALBI组患者间性别、年龄、肿瘤部位、肿瘤大小、CA19-9水平、手术方式、肿瘤T分期、血管侵袭、淋巴结转移、血管受侵、内脏神经受侵、联合脏器切除等临床病理因素差异无统计学意义(均P>0.05),但高ALBI组术前因胆管梗阻行胆道引流比例明显高于低ALBI组(P=0.008)(表1)。
表1 ALBI 评分与临床病理特征的关系[n(%)]
Table 1 Relations of ALBI with the clinicopathological features[n (%)]
2.3 ALBI 评分、CA19-9 与患者远期生存的关系
全组患者平均随访时间为32(4~113)个月,高ALBI 组5 年生存率明显低于低A L B I 组的患者(22.2% vs.42.3%,P=0.036)。本院实验室CA19-9常规参考值为≤37 IU/mL,故本研究将CA19-9值>37 IU/mL的患者作为高CA19-9组,而≤37 IU/mL的患者作为低CA19-9组,结果显示,高CA19-9组患者的5年生存率明显低于低CA19-9组(24.1% vs.44.2%,P=0.035)。
尽管ALBI评分和CA19-9之间并无直接关联(P=0.983),但以上结果显示了两者均对对胰腺癌患者预后有一定评估价值。因此,根据患者ALBI 与CA19-9 的高低分为4 组:高A L B I+高CA19-9患者25例(双高组),高ALBI+低CA19-9患者20例(高ALBI组),低ALBI+高CA19-9患者29例(高CA19-9组),低ALBI+低CA19-9患者23例(双低组)。4组的5年生存率分别为12.0%、35.0%、34.5%、52.2%,将高ALBI组和高CA19-9组合(单高组)并进行分析,双高组患者5年生存率明显低于单高组和双低组(12.0% vs.34.7%,P=0.038;12.0% vs.52.2%,P=0.003),而单高组与双低组之间差异无统计学意义(34.7% vs.52.2%,P=0.159)(表2)。
表2 不同参数与患者5 年生存率的关系(%)
Table 2 Relationships between different parameters and the 5-year survival rate of the patients (%)
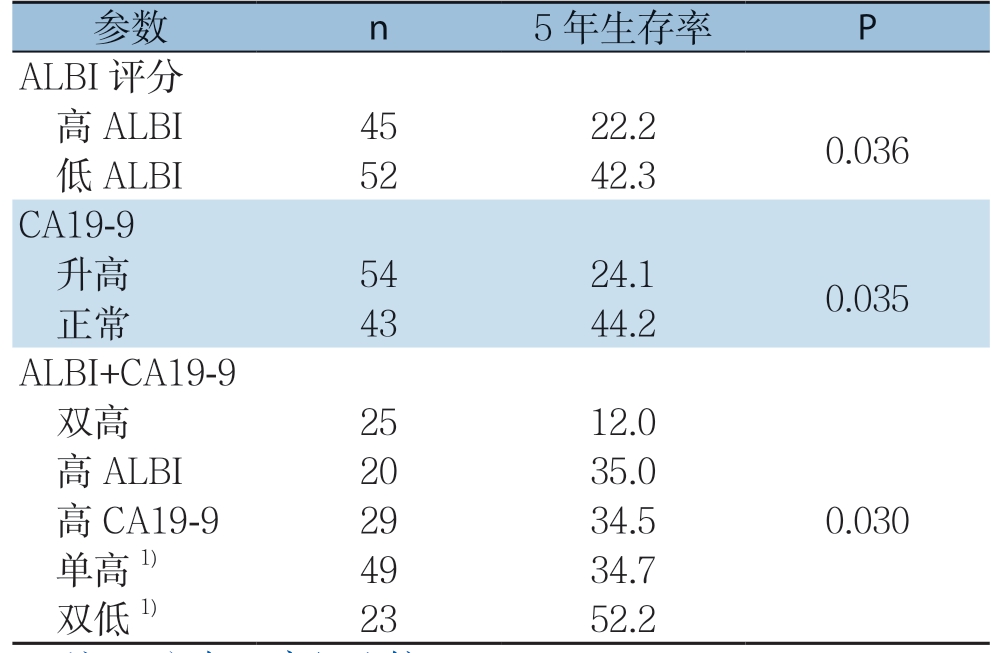
注:1)与双高组比较,P<0.05
Note: 1) P<0.05 vs.double-high group
2.4 影响患者远期生存因素的单因素和多因素分析
单因素分析显示,淋巴结转移(P=0.002)、血管受侵(P=0.022)、神经受侵(P=0.036)、ALBI评分与CA19-9联合应用(P=0.001)与胰腺癌患者远期生存密切相关(表3)。多因素分析显示,淋巴结转移(P=0.012)、ALBI评分与CA19-9联合(P=0.009)是影响胰腺癌患者预后的独立危险因素(表4)。
表3 胰腺癌患者远期生存因素的单因素分析
Table 3 Univariate analysis of factors for long-term survival of pancreatic cancer patients
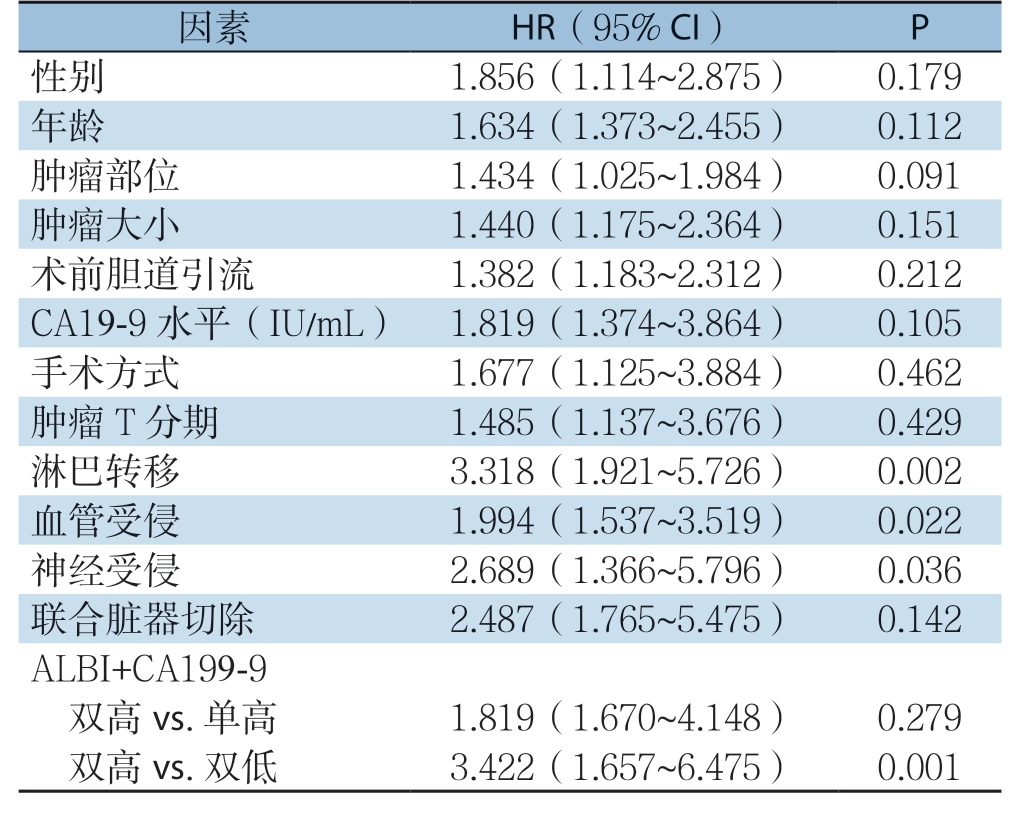
因素 HR(95% CI) P性别 1.856(1.114~2.875) 0.179年龄 1.634(1.373~2.455) 0.112肿瘤部位 1.434(1.025~1.984) 0.091肿瘤大小 1.440(1.175~2.364) 0.151术前胆道引流 1.382(1.183~2.312) 0.212 CA19-9 水平(IU/mL) 1.819(1.374~3.864) 0.105手术方式 1.677(1.125~3.884) 0.462肿瘤T 分期 1.485(1.137~3.676) 0.429淋巴转移 3.318(1.921~5.726) 0.002血管受侵 1.994(1.537~3.519) 0.022神经受侵 2.689(1.366~5.796) 0.036联合脏器切除 2.487(1.765~5.475) 0.142 ALBI+CA199-9双高vs.单高 1.819(1.670~4.148) 0.279双高vs.双低 3.422(1.657~6.475) 0.001
表4 胰腺癌患者远期生存因素的多因素分析
Table 4 Multivariate analysis of factors for long-term survival of pancreatic cancer patients
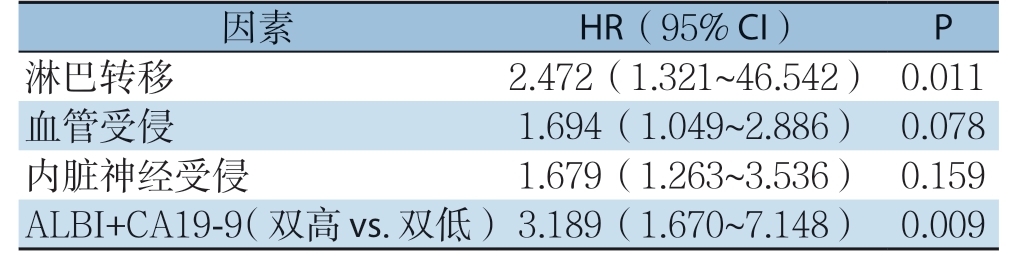
因素 HR(95% CI) P淋巴转移 2.472(1.321~46.542) 0.011血管受侵 1.694(1.049~2.886) 0.078内脏神经受侵 1.679(1.263~3.536) 0.159 ALBI+CA19-9(双高vs.双低)3.189(1.670~7.148) 0.009
3 讨 论
本研究表明ALBI评分对评估胰腺癌患者的预后具有重要临床价值,ALBI评分依靠血清白蛋白水平和总胆红素浓度计算获得,其与血清白蛋白水平呈负相关。较高的ALBI评分反映了血清胆红素浓度的升高,这通常提示肝功能障碍[14]。胰腺癌患者特别是肿瘤位于胰头部,多数合并胆道梗阻,此类患者势必存在胆红素浓度升高,而术前胆道引流是胰头癌合并梗阻性黄疸的常规治疗方法[15]。如本研究所示,高ALBI评分胰腺癌患者术前进行胆道引流明显高于低ALBI评分的患者。然而,完成胆汁引流操作的患者术前血清胆红素浓度仍明显高于无胆汁引流的患者。因此,术前高血清胆红素浓度患者的营养状况较差,即便在胆道引流后也是如此,这可能是本研究患者的预后不佳的原因之一。需要指出的是,本研究在进行预后因素的分析中发现术前胆道引流并不能改善胰腺癌远期预后,与既往文献[16-18]报道一致。
另一方面,血清白蛋白是肝脏合成的最主要血浆蛋白,是用来评估患者营养状况的标准指标。由于肿瘤阻塞胆管进而造成肝脏合成功能受到抑制,白蛋白水平存在不同程度的下降,而患者自身营养状况将直接影响胰腺癌患者的预后[19]。近年来研究证实炎症和恶性肿瘤之间的相关性[20-25],肿瘤引起的全身炎症反应及其微环境在肿瘤的发生发展过程中起重要作用[26]。目前已有中性粒细胞与淋巴细胞比率、血小板与淋巴细胞比率和CRP水平等指标与胰腺癌患者的预后相关的报道[27-28],而炎症反应通常抑制白蛋白的合成,在胰腺癌患者中经常出现血清白蛋白水平显著降低。因此,ALBI评分同时反映了患者的营养状况和炎症状况,且两者都与胰腺癌患者的预后密切相关。此外,由于肿瘤阻塞胆管而导致的胆汁排泄障碍会导致吸收不良,从而对患者的营养状况产生不利影响。总的来说,ALBI评分反映了诸如营养状况、炎症反应和免疫功能等与患者个体相关的因素,而这些因素又与恶性肿瘤的进展和预后密切相关。
ALBI评分反映的是患者个体相关性因素,而CA19-9是评估胰腺癌预后最主要的肿瘤标志物[6, 29-30]。由此推测在评估胰腺癌患者预后时,ALBI评分和血清CA19-9两者联合应用可能优于仅使用ALBI评分或血清CA19-9,本研究结果提供的数据有力地支持了这一假设,即当ALBI评分和CA19-9同时升高时,5年生存率明显低于CA19-9正常或(和)低ALBI评分组,而其它各组间比较并无差异;多因素分析显示,ALBI与CA19-9评分的联合应用是胰腺癌患者独立的预后指标,表明这两项指标可为胰腺癌的诊疗提供重要的临床参考价值。
尽管本研究试图避免各种可能性偏倚,如通过手术当日获取CA19-9与ALBI评分,而非入院初期结果,最大限度的避免因术前胆道引流造成的数据误差,但仍存在一定的局限性。一方面,由于目前尚无关于胰腺癌ALBI评分的分界值,本文中使用的分界值是通过肝癌研究确定的[31]。因此,计算胰腺癌患者ALBI评分的最佳分界仍有待确定。事实上,本研究仅包括2例ALBI 3级患者,仍不能完全反应临床实际工作中胰腺癌患者真实情况。另一方面,本研究纳入的患者数量较少,其结果必须经过大规模、前瞻性、随机、对照试验的证实。
综上所述,本研究结果提示A L B I 评分和CA19-9联合应用对评估胰腺癌患者预后的潜力。ALBI评分中所需指标检测具有简便、易获取、可重复的优势,可作为评价胰腺癌患者预后的有效临床标志物。
[1] Canto MI, Almario JA, Schulick RD, et al.Risk of Neoplastic Progression in Individuals at High Risk for Pancreatic Cancer Undergoing Long-term Surveillance[J].Gastroenterology,2018,155(3):740-751.doi: 10.1053/j.gastro.2018.05.035.
[2] Oettle H, Neuhaus P, Hochhaus A, et al.Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial[J].JAMA, 2013, 310(14):1473-1481.doi: 10.1001/jama.2013.279201.
[3] Neoptolemos JP, Stocken DD, BassiC, et al.Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial[J].JAMA, 2010, 304(10):1073-1081.doi: 10.1001/jama.2010.1275.
[4] Uesaka K, Boku N, Fukutomi A, et al.Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, openlabel, randomised, non-inferiority trial (JASPAC 01)[J].Lancet,2016, 388(10041):248-257.doi: 10.1016/S0140-6736(16)30583-9.
[5] 隋宇航, 孙备.胰腺癌临床研究的热点问题[J].中国普通外科杂志, 2019, 28(3):255-259.doi:10.7659/j.issn.1005-6947.2019.03.001.Sui YH, Sun B.Hot issues in clinical research of pancreatic cancer[J].Chinese Journal of General Surgery, 2019, 28(3):255-259.doi:10.7659/j.issn.1005-6947.2019.03.001.
[6] Tempero MA, Malafa MP, Al-Hawary M, et al.Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology[J].J Natl Compr Canc Netw, 2017,15(8):1028-1061.doi: 10.6004/jnccn.2017.0131.
[7] Lee HS, Jang CY, Kim SA, et al.Combined use of CEMIP and CA 19-9 enhances diagnostic accuracy for pancreatic cancer[J].Sci Rep, 2018, 8(1):3383.doi: 10.1038/s41598-018-21823-x.
[8] Fujita K, Nomura T, Morishita A, et al.Albumin-Bilirubin Score Differentiates Liver Fibrosis Stage and Hepatocellular Carcinoma Incidence in Chronic Hepatitis B Virus Infection: A Retrospective Cohort Study[J].Am J Trop Med Hyg, 2019, 101(1):220-225.doi:10.4269/ajtmh.19-0129.
[9] Johnson PJ, Berhane S, Kagebayashi C, et al.Assessment of liver function in patients with hepatocellular carcinoma: a new evidencebased approach-the ALBI grade[J].J Clin Oncol, 2015, 33(6):550-558.doi: 10.1200/JCO.2014.57.9151.
[10] Elshaarawy O, Alkhatib A, Elhelbawy M, et al.Validation of modified albumin-bilirubin-TNM score as a prognostic model to evaluate patients with hepatocellular carcinoma[J].World J Hepatol,2019, 11(6):542-552.doi: 10.4254/wjh.v11.i6.542.
[11] Kanda M, Tanaka C, Kobayashi D, et al.Preoperative Albumin-Bilirubin Grade Predicts Recurrences After Radical Gastrectomy in Patients with pT2-4 Gastric Cancer[J].World J Surg, 2018,42(3):773-781.doi: 10.1007/s00268-017-4234-x.
[12] Miwa T, Kanda M, Tanaka C, et al.Albumin-Bilirubin Score Predicts Tolerability to Adjuvant S-1 Monotherapy after Curative Gastrectomy[J].J Gastric Cancer, 2019, 19(2):183-192.doi:10.5230/jgc.2019.19.e15.
[13] Allen PJ, Kuk D, Castillo CF, et al.Multi-institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma[J].Ann Surg, 2017, 265(1):185-191.doi: 10.1097/SLA.0000000000001763.
[14] Pinato DJ, Sharma R, Citti C, et al.The albumin-bilirubin grade uncovers the prognostic relationship between hepatic reserve and immune dysfunction in HIV-associated hepatocellular carcinoma[J].Aliment Pharmacol Ther, 2018, 47(1):95-103.doi: 10.1111/apt.14356.
[15] Kamisawa T, Wood LD, Itoi T, et al.Pancreatic cancer[J].Lancet,2016, 388(10039):73-85.doi: 10.1016/S0140-6736(16)00141-0.
[16] Trujillo-Ochoa JL, Corral-Jara KF, Charles-Niño CL, et al.Conjugated Bilirubin Upregulates TIM-3 Expression on CD4+CD25+ T Cells: Anti-Inflammatory Implications for Hepatitis A Virus Infection[J].Viral Immunol, 2018, 31(3):223-232.doi:10.1089/vim.2017.0103.
[17] 黄耿文, 宁彩虹, 申鼎成, 等.《日本胰腺协会胰腺癌临床实践指南(2016)》解读[J].中国普通外科杂志, 2017, 26(9):1093-1096.doi:10.3978/j.issn.1005-6947.2017.09.003.Huang GW, Ning CH, Shen DC, et al.Interpretation of Clinical Practice Guidelines for Pancreatic Cancer 2016 from the Japan Pancreas Society[J].Chinese Journal of General Surgery, 2017,26(9):1093-1096.doi:10.3978/j.issn.1005-6947.2017.09.003.
[18] 刘江, 吉顺荣, 徐近, 等.临界可切除胰腺癌的诊疗策略[J].中国普通外科杂志, 2017, 26(9):1089-1092.doi:10.3978/j.issn.1005-6947.2017.09.002.Liu J, Ji SX, Xu J, et al.Diagnosis and treatment strategies for borderline resectable pancreatic cancer[J].Chinese Journal of General Surgery, 2017, 26(9):1089-1092.doi: 10.3978/j.issn.1005-6947.2017.09.002.
[19] 梁夏宜, 刘军杰, 王夺.胰腺导管腺癌的治疗研究进展[J].中国普通外科杂志, 2018, 27(9):1173-1179.doi:10.7659/j.issn.1005-6947.2018.09.014.Liang XY, Liu JJ, Wang D.Research progress of treatment for pancreatic ductal adenocarcinoma[J].Chinese Journal of General Surgery,2018,27(9):1173-1179.doi:10.7659/j.issn.1005-6947.2018.09.014.
[20] Lee SH, Chung MJ, Kim B, et al.The Significance of the Prognostic Nutritional Index for All Stages of Pancreatic Cancer[J].Nutr Cancer, 2017, 69(3):512-519.doi:10.1080/01635581.2016.1250921.
[21] Morinaga S, Murakawa M, Katayama Y, et al.Glasgow Prognostic Score Predicts Clinical Outcomes in Patients with Pancreatic Cancer Undergoing Adjuvant Gemcitabine Monotherapy After Curative Surgery[J].Anticancer Res, 2015, 35(9):4865-4870.
[22] Haruki K, Shiba H, Shirai Y, et al.The C-reactive Protein to Albumin Ratio Predicts Long-Term Outcomes in Patients with Pancreatic Cancer After Pancreatic Resection[J].World J Surg,2016, 40(9):2254-2260.doi: 10.1007/s00268-016-3491-4.
[23] Tokunaga R, Sakamoto Y, Nakagawa S, et al.Comparison of systemic inflammatory and nutritional scores in colorectal cancer patients who underwent potentially curative resection[J].Int J Clin Oncol, 2017, 22(4):740-748.doi: 10.1007/s10147-017-1102-5.
[24] Kumar R, Geuna E, Michalarea V, et al.The neutrophil-lymphocyte ratio and its utilisation for the management of cancer patients in early clinical trials[J].Br J Cancer, 2015, 112(7):1157-1165.doi:10.1038/bjc.2015.67.
[25] Nielsen MF, Mortensen MB, Detlefsen S.Key players in pancreatic cancer-stroma interaction: Cancer-associated fibroblasts, endothelial and inflammatory cells[J].World J Gastroenterol, 2016, 22(9):2678-2700.doi: 10.3748/wjg.v22.i9.2678.
[26] Singh R, Mishra MK, Aggarwal H.Inflammation, Immunity,and Cancer[J].Mediators Inflamm, 2017, 2017:6027305.doi:10.1155/2017/6027305.
[27] Asaoka T, Miyamoto A, Maeda S, et al.Prognostic impact of preoperative NLR and CA19-9 in pancreatic cancer[J].Pancreatology, 2016, 16(3):434-440.doi: 10.1016/j.pan.2015.10.006.
[28] Kim WJ, Lim TW, Park PJ, et al.Prognostic impact of the combination of the neutrophil-to-lymphocyte ratio and serum carbohydrate antigen 19-9 in patients with pancreas head cancer[J].ANZ J Surg, 2019, 89(7/8):E302-307.doi: 10.1111/ans.15029.
[29] Epelboym I, Zenati MS, Hamad A, et al.Analysis of Perioperative Chemotherapy in Resected Pancreatic Cancer: Identifying the Number and Sequence of Chemotherapy Cycles Needed to Optimize Survival[J].Ann Surg Oncol, 2017, 24(9):2744-2751.doi:10.1245/s10434-017-5975-3.
[30] Rieser CJ, Zenati M, Hamad A, et al.CA19-9 on Postoperative Surveillance in Pancreatic Ductal Adenocarcinoma: Predicting Recurrence and Changing Prognosis over Time[J].Ann Surg Oncol,2018, 25(12):3483-3491.doi: 10.1245/s10434-018-6521-7.
[31] Chan AW, Chan RC, Wong GL, et al.New simple prognostic score for primary biliary cirrhosis: Albumin-bilirubin score[J].J Gastroenterol Hepatol, 2015, 30(9):1391-1396.doi: 10.1111/jgh.12938.
