1982年,日本学者Watari等[1]用电子显微镜和自发荧光技术在小鼠胰腺中观察到一种与肝脏星状细胞(hepatic stellate cells,HSC)很相似,并且具有维生素A储存功能的细胞。1998年,Apte等[2]使用梯度离心法分离出大鼠静息态胰腺星状细胞(pancreatic stellate cells,PSC)。Bachem等[3]通过组织块培养法分离出激活态PSC。至此,这两个开创性的报告成功完成了这种细胞的分离、培养,并最终将其命名为PSC。这种间质细胞具有许多与HSC相似的生物学特性。因此之前关于HSC生物学机制[4-5]的研究也有助于进一步研究它的生物学行为。同时,在胰腺肿瘤及胰腺炎症的细胞微坏境中,持续激活的PSC发挥的作用也引起越来越多的关注,这是胰腺疾病研究领域的一颗冉冉升起的新星。因此,深入了解PSC的生物学特性,可以为进一步探索各种胰腺疾病中PSC发挥的功能奠定基础,同时为PSC相关靶向治疗提供新的思路。
1 PSC 的功能异质性
1.1 PSC 功能异质性的起源
目前,细胞谱系的追踪研究已经证实了HSC是来源于间充质细胞并且是由中胚层起源进化而来[6]。与此同时,转录组学和蛋白质组学分析的结果表明,尽管HSC和PSC存在器官特异性,但两者有着显著的相似性[7]。由此,笔者推测PSC可能来源于中胚层细胞。但在最近的研究中,Scarlett等[8]发现了在慢性胰腺炎和胰腺导管腺癌(pancreatic ductal adenocarcinoma,PDAC)中一定比例的PSC被认为来源于骨髓。另外一项涉及二丁基氯化物引发慢性胰腺炎的研究[9]也表明了骨髓可能是PSC另一种潜在来源。还有研究[10]报道,CCR2+单核细胞可以通过MCP-1/CCR2途径迁移到胰腺并分化成PSC。以上这些研究都阐明了骨髓作为PSC来源的可能性。因而,在某种程度上,PSC之所以具有功能异质性,可能是因为其一部分细胞来源于骨髓造血细胞而另一部分则来源于中胚层细胞。因此,今后对PSC来源的深入探索可以帮助我们发现这类细胞的本质,从而进一步推测出PSC更多的功能及其临床意义。
1.2 PSC 的细胞标志物
近年来,很多癌症生物学研究集中在肿瘤干细胞(cancer stem cells,CSC)领域[11-13]。CSC是一种具有启动和维持肿瘤形成的能力的干细胞[14],其可以基于其细胞表面标志物的不同来进行分离和筛选[11-13]。综合PSC的起源和CSC的异质性,最近提出了PSC的异质性,即PSC基于细胞表面标志物的不同而有了不同的细胞亚群并在胰腺组织的病理生理学变化中发挥着不同的生物学功能。如Ikenaga等[15]通过体外共培养实验表明,CD10阳性的PSC比CD10阴性的PSC更能促进胰腺癌细胞SUIT-2和Panc-1的增殖、生长和转移。而且,相对于CD10阴性的PSC,CD10阳性的PSC促进PCC皮下成瘤的能力更强。同时,Mizutani等[16]发现了Meflin,这是一种由PSC表达的糖基磷脂酰肌醇锚定蛋白,它与PDAC的疾病进展及胰腺癌患者的预后密切相关。在PDAC小鼠模型中,Meflin缺乏或低表达会导致明显的肿瘤进展,而在PSC中过表达Meflin则会抑制肿瘤的生长,这一事实阐明了Meflin是抑制PDAC进展的PSC的标记物。Farrow等[17]对肿瘤来源的PSC进行了分离和鉴定,发现了S100A4是活化的PSC的一种独特的生物标志物。它是胰腺肿瘤微环境的重要组分,并调控着胰腺癌的进展。Fujiwara等[18]发现了PSC另一种标记物,即CD271。通过研究,他们发现CD271阳性的PSC多存在于胰腺肿瘤的边缘,而非肿瘤中心。而当将这类PSC与胰腺癌细胞长期共培养后或者当它向肿瘤中心移动后,其CD271的表达量会下降。结合其他相关研究,最终他们得出结论,CD271阳性的PSC多出现在胰腺癌发生的早期,CD271阳性的PSC占比越高,胰腺癌患者的预后愈加良好。以上这些标志物的发现证明了PSC有着不同的细胞亚群,并具备着不同的生物学功能。但是还需要对PSC生物标志物进行更进一步地探索,这对于鉴定PSC的功能异质性尤为重要,甚至能以此来追溯胰腺癌的高恶性程度和不良预后的本质。同时,在进一步的研究中,这些生物标志物也许可以作为诊断和治疗胰腺癌新的靶点。
1.3 PSC 的功能学分型
Tjomsland等[19]从不同人类的PDAC分离出不同的PSC群体,进而研究PSC条件培养基对胰腺癌细胞的影响。他们发现不同的PSC群体在分泌肝细胞生长因子(hepatocyte growth factor,HGF)的能力方面表现出广泛的变异。而相对于低水平分泌HGF的PSC,高水平分泌HGF的PSC对胰腺癌细胞的生存和增殖的促进作用更强。因此,HGF介导的肿瘤-基质相互作用可能是PSC产生功能异质性的成因。同时,Öhlund等[20]揭示了PSC的另一个有意义的亚群,在2D和3D的胰腺癌细胞-PSC共培养模型中,距离肿瘤细胞较远的P S C 亚群分泌更少的α-平滑肌肌动蛋白(alpha smooth muscle actin,α-SMA),转而表达更多的白细胞介素6和其他炎症相关介质,这一事实直接证明了包括PSC在内的癌症相关成纤维细胞(cancerassociated fibroblasts,CAF)具有功能异质性。此外,不同组织中的间充质细胞和成纤维细胞也表现出不同的表型,Strobel等[21]分离了来自健康个体、慢性胰腺炎患者和PDAC患者的胰腺组织,并培养出原代PSC,并在低血清环境中进行比较分析。结果显示,从不同疾病背景的个体中分离培养出来的PSC有着不同的表型,这可能是原始组织中基质活性的不同所造成的。最近的几项研究也佐证了这一观点,Bynigeri等[22]发现了细胞外基质(extracellular matrix,ECM)的组成显着影响PSC的基因表达模式,从而导致PSC的功能异质性。Neuzillet等[23]则通过转录组学分析和免疫荧光染色法证实了PDAC中存在PSC的多种功能亚型,不同的亚型在波形蛋白和α-SMA的表达量、增殖率以及免疫相关特征上有着显著的差异。总的来说,PSC可以与胰腺实质细胞相互作用,并根据所处细胞微坏境的不同而具有功能学上的差异。因此,对PSC在不同疾病背景下的详细表征的进一步研究有助于阐明胰腺实质细胞和间质细胞相互作用的具体机制,同时可以引导胰腺疾病精准化治疗的方向。
2 PSC 在外泌体领域的研究进展
2.1 外泌体的定义
外泌体是由活细胞分泌的一种直径在40~100 nm之间,且具有生物活性的囊泡。它可以存在于各种体液当中,如血液、组织液以及尿液等,并能介导细胞之间的物质交换、信号传导和信息交流,同时,它能以细胞之间的媒介的角色在人体各种生理病理过程中发挥着重要作用[24-29]。外泌体中含有多种细胞衍生的生物活性分子,包括microRNA、脂质以及蛋白质等。当组织发生癌变时,病理状态的细胞可以比正常细胞分泌更多的外泌体。这些外泌体可以将信号传导分子运输到特定的细胞和组织上,形成有利于肿瘤生长和侵袭的微环境,从而调控肿瘤的生长[30]。近年来,microRNA成为外泌体领域研究的热点,microRNA是一类内源性非编码的小分子RNA,它可以调控细胞的增殖分化、细胞凋亡、免疫应答反应、组织修复以及间质纤维化等多种生物过程,并且相比于循环内microRNA,外泌体内miRNA由于被外泌体的脂质双层膜包被,因此能更稳定的发挥功能[31-36]。同时,外泌体内的其他信号传导分子,如蛋白质等也参与了多种疾病的进展过程[37-40]。因此,基于外泌体在生物功能调控方面的多种作用,外泌体可能成为未来治疗疾病的潜在标志物及治疗靶标。
2.2 PSC 衍生的外泌体对胰腺癌的影响
PSC衍生的外泌体能够促进胰腺癌细胞的生长、增殖、侵袭和转移并增加其对化疗药物的抵抗能力。最近的很多研究说明了这一点,如Takikawa等[36]报道了PSC衍生的外泌体含有多种microRNA,它们对胰腺癌细胞的趋化因子基因表达、迁移、增殖有着显著的促进作用。同时,Leca等[37]的研究也表明PSC衍生的膜联蛋白A6阳性的外泌体能够促进胰腺癌侵袭和转移。Zhao等[38]研究者阐明了PSC衍生的外泌体可以向营养素缺乏的胰腺癌细胞提供代谢物,抑制线粒体氧化磷酸化,并增加癌细胞中的糖酵解和谷氨酰胺依赖性的还原羧化,从而使胰腺癌细胞具备更强的生存能力。此外,Richards等[39]通过实验阐明,即使用吉西他滨处理之后,包括PSC在内的CAF释放的外泌体仍然能够提高受体上皮癌细胞的增殖和存活能力。由此可见,在胰腺癌中,PSC衍生的外泌体介导的细胞间信息传导和物质交换可以给提供肿瘤细胞更好的生存、增殖和转移的条件,从而加速胰腺癌的病情进展。
2.3 由外泌体介导的胰腺癌细胞与PSC 之间的相互作用
目前,很多研究集中在胰腺肿瘤微环境的形成上,如Masamune等[41]证明了胰腺癌细胞衍生的外泌体可以使P S C 中细胞外信号调节激酶(extracellular signal-regulated kinase,ERK)和丝氨酸/苏氨酸激酶(serine/threonine kinase,Akt)活化。并且增加其α-SMA和纤维化相关基因的mRNA的表达以及促进I型前胶原C肽的产生,从而激活PSC。与此同时,激活态PSC又可以衍生多种外泌体来增强胰腺癌细胞的增殖和侵袭能力。Zhang等[42]阐明了胰腺癌细胞衍生的外泌体可以通过激活多种信号传导途径来大量募集PSC,而这些被募集而来PSC又可以进一步促进胰腺癌细胞的远处转移。由此可见,胰腺癌细胞与PSC以外泌体为媒介形成了肿瘤发生发展的微环境,进而调控胰腺癌的疾病进展[43-45](图1)。

图1 胰腺癌细胞与PSC 以外泌体为媒介的肿瘤微环境
Figure 1 Tumor microenvironment mediated by exosomes of pancreatic cancer cells and PSCs
2.4 激活态PSC 衍生的外泌体及其去活化对慢性胰腺炎的影响
慢性胰腺炎是由各种发病诱因引起胰腺组织和功能产生持续变化的一种慢性炎症性疾病。随着疾病的发展,胰腺组织不断发生不可逆的间质纤维组织增生和玻璃样改变,严重时可能发生癌变[46-48]。近年来的研究发现激活态的PSC可以衍生多种外泌体并以此调控慢性胰腺炎的进展,如Charrier等[49]发现了静息态的PSC经历表型和功能的改变成为激活态PSC的这一过程是受结缔组织生长因子(connective tissue growth factor,CTGF)调节的。当PSC 被CTGF 激活后,其可以表达microRNA-21以产生的更多的CTGF,所以CTGF与microRNA-21之间还存在着正反馈通路(图2)。而microRNA-21和CTGF均存在于PSC衍生的外泌体中,所以它们之间的相互作用是PSC衍生的外泌体调节胰腺纤维化进程并影响慢性胰腺炎进展的方式。目前还发现几种microRNA可以调控纤维化进程,如microRNA-33a能通过Smads信号通路来引发组织纤维化,microRNA-10a也具有相似的作用[50-51]。而microRNA-148a和microRNA-let-7d-5p则可以抑制PSC的活化和胰腺纤维化 [52-54]。不过这些microRNA的表达与PSC衍生的外泌体之间的关系以及是否有更多的外泌体在胰腺纤维化这一关键环节发挥着重要作用则亟需进一步研究。但是基于目前的研究,笔者了解到PSC的激活是胰腺纤维化进程中的关键步骤[55]。因此慢性胰腺炎的治疗重点应该集中在PSC的去活化机制上,Xue等[56-57]发现辅酶Q10的预处理和后处理均能降低PSC引发的氧化应激反应,组织学变化和胶原蛋白沉积,从而抑制PSC的活化。Xu等[58]发现了转化生长因子β(transforming growth factor β,TGF-β)是PSC介导的胰腺组织纤维化的关键刺激剂,并且更多的研究还探明了TGF-β1可能受到PI3K/Akt途径的调控,但其串联的具体机制及重要性亟需进一步研究[59]。总之,激活态PSC衍生的外泌体和其去活化机制在慢性胰腺炎的病情进展中扮演着非常重要的角色,随着对其认识和研究的深入,有望为慢性胰腺炎的临床治疗提供新的方法和策略。
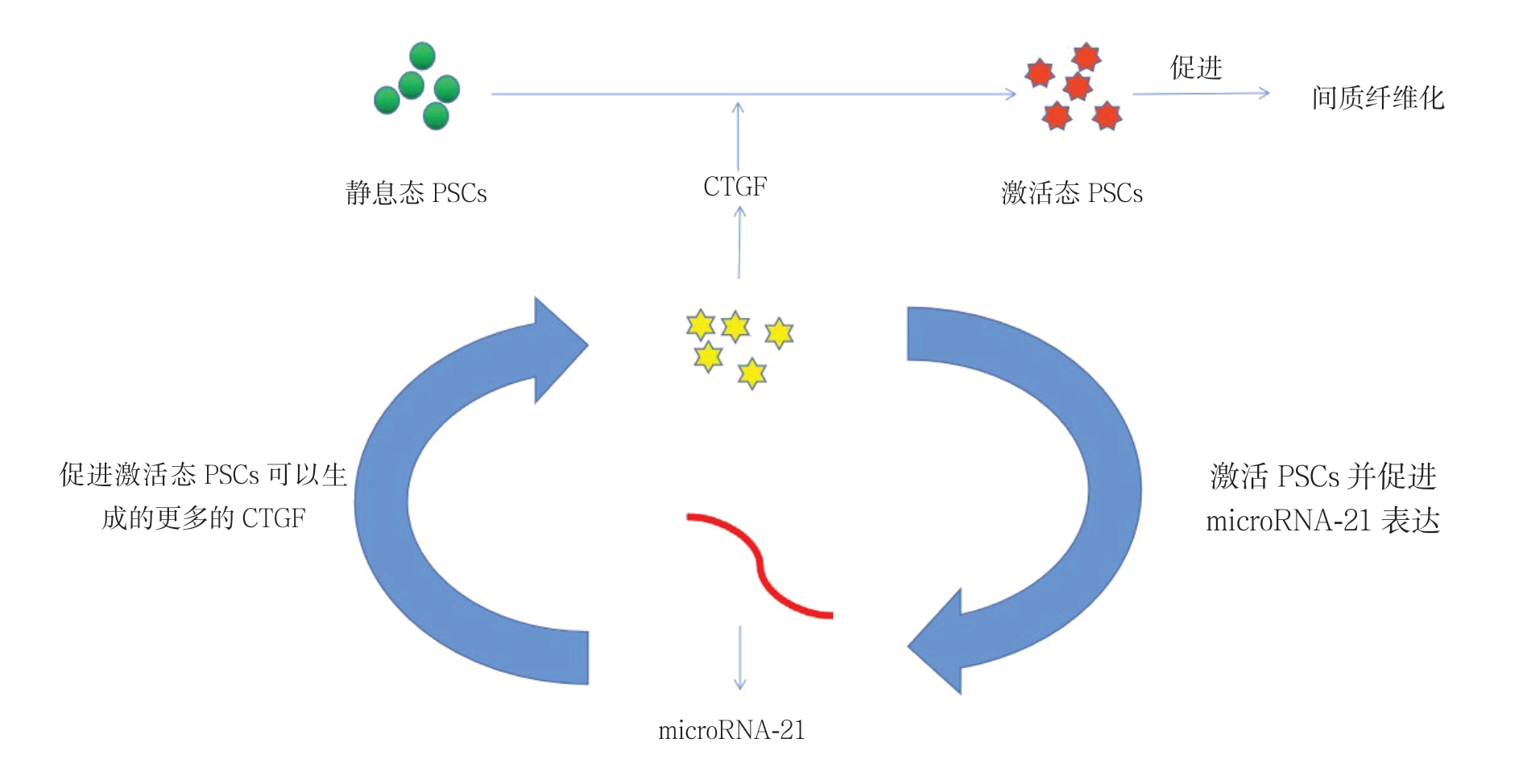
图2 CTGF 与microRNA-21 构成正反馈通路促进胰腺纤维化
Figure 2 CTGF and microRNA-21 forming a positive feedback pathway to promote pancreatic fibrosis
3 总结与展望
综上所述,PSC因其细胞标志物的不同和功能学的差异有着广泛变异的亚群,这些亚群具有功能异质性。因此,使特定亚群的PSC转为静息态可以阻断ECM大量分泌,从而切断PSC与胰腺癌细胞的相互作用,抑制肿瘤进展并改善疾病预后。同时,PSC的相关外泌体介导的细胞间信息传递和物质交换则以媒介的形式同胰腺实质细胞以及PSC形成了胰腺癌和慢性胰腺炎发生发展的微环境,所以阻断特定的外泌体介导的信息传导和物质交换的通路可以阻断胰腺癌和慢性胰腺炎的疾病进展。随着对PSC功能异质性和其相关外泌体的研究的深入,PSC激活途径相关的靶向治疗的方法也许即将问世。当然,为了能够更好地探索各种胰腺疾病的发生和发展的机制,PSC永生化的问题是值得积极探索的方向[60]。这一新技术将增进对胰腺癌细胞生物学行为的认识。总之,对于PSC领域的进一步研究可能为胰腺疾病治疗提供重大突破。
[1] Watari N, Hotta Y, Mabuchi Y.Morphological studies on a vitamin A-storing cell and its complex with macrophage observed in mouse pancreatic tissues following excess vitamin A administration[J].Okajimas Folia Anat Jpn, 1982, 58(4/6):837-858.doi:10.2535/ofaj1936.58.4-6_837.
[2] Apte MV, Haber PS, Applegate TL, et al.Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture[J].Gut,1998, 43(1):128-133.doi: 10.1136/gut.43.1.128.
[3] Bachem MG, Schneider E, Gross H, et al.Identification, culture,and characterization of pancreatic stellate cells in rats and humans [J].Gastroenterology, 1998, 115(2):421-432.doi: 10.1016/s0016-5085(98)70209-4.
[4] 赵鸽, 狄首印, 翟蒙恩, 等.血管钠肽抑制肝纤维化的实验研究[J].中国普通外科杂志, 2011, 20(8):830-834.Zhao G, Di SY, Zhai ME, et al.Study of inhibitory effects of vasonatrin peptide on liver fibrosis[J].Chinese Journal of General Surgery, 2011, 20(8):830-834.
[5] 叶轲, 白宁, 周乐杜, 等.紫衫醇对大鼠肝纤维化的抑制作用及机制研究[J].中国普通外科杂志, 2017, 26(9):1155-1161.doi:10.3978/j.issn.1005-6947.2017.09.012.Ye K, Bai N, Zhou LD, et al.Inhibitory effect of paclitaxel on hepatic fibrosis in rats and its mechanism[J].Chinese Journal of General Surgery, 2017, 26(9):1151-1161.doi:10.3978/j.issn.1005-6947.2017.09.012.
[6] Cassiman D, Barlow A, Vander Borght S, et al.Hepatic stellate cells do not derive from the neural crest[J].J Hepatol, 2006, 44(6):1098-1104.doi: 10.1016/j.jhep.2005.09.023.
[7] Paulo JA, Kadiyala V, Banks PA, et al.Mass spectrometry-based quantitative proteomic profiling of human pancreaticand hepatic stellate cell lines[J].Genomics Proteomics Bioinformatics, 2013,11(2):105-113.doi: 10.1016/j.gpb.2013.01.009.
[8] Scarlett CJ, Colvin EK, Pinese M, et al.Recruitment and activation of pancreatic stellate cells from the bone marrow in pancreatic cancer: a model of tumor-host interaction[J].PLoS One, 2011,6(10):e26088.doi: 10.1371/journal.pone.0026088.
[9] Sparmann G, Kruse ML, Hofmeister-Mielke N, et al.Bone marrow-derived pancreatic stellate cells in rats[J].Cell Res, 2010,20(3):288-298.doi: 10.1038/cr.2010.10.
[10] Ino K, Masuya M, Tawara I, et al.Monocytes infiltrate the pancreas via the MCP-1/CCR2 pathway and differentiate into stellate cells[J].PLoS One, 2014, 9(1):e84889.doi: 10.1371/journal.pone.0084889.
[11] 成蕾, 肖云峰, 李孝琼, 等.FH535对结直肠癌肿瘤干细胞自我更新及侵袭迁移的抑制作用及机制[J].中国普通外科杂志, 2018,27(10):1288-1294.doi:10.7659/j.issn.1005-6947.2018.10.011.Cheng L, Xiao YF, Li XQ, et al.Inhibitory effect of FH535 on selfrenewal, migration and invasion of colorectal cancer stem cells and the mechanism[J].Chinese Journal of General Surgery, 2018,27(10):1288-1294.doi:10.7659/j.issn.1005-6947.2018.10.011.
[12] 董华英, 王伟, 陈元文, 等.Luminal A型和HER-2阳性乳腺癌的肿瘤干细胞生物学行为比较[J].中国普通外科杂志, 2017,26(11):1431-1438.doi:10.3978/j.issn.1005-6947.2017.11.010.Dong HY, Wang WY, Chen YW, et al.Comparison of biological behaviors in cancer stem cells derived from Luminal A and HER-2-positive breast cancer[J].Chinese Journal of General Surgery, 2017,26(11):1431-1438.doi:10.3978/j.issn.1005-6947.2017.11.010.
[13] 洪乐, 肖卫东.microRNA调控胰腺癌干细胞的作用研究进展[J].中国普通外科杂志, 2019, 28(9):1137-1142.doi:10.7659/j.issn.1005-6947.2019.09.016.Hong L, Xiao WD.Research progress of the role of microRNAs in regulating pancreatic cancer stem cells[J].Chinese Journal of General Surgery, 2019, 28(9):1137-1142.doi:10.7659/j.issn.1005-6947.2019.09.016.
[14] O'Brien-Ball C, Biddle A.Reprogramming to developmental plasticity in cancer stem cells[J].Dev Biol, 2017, 430(2):266-274.doi: 10.1016/j.ydbio.2017.07.025.
[15] Ikenaga N, Ohuchida K, Mizumoto K, et al.CD10+ pancreatic stellate cells enhance the progression of pancreatic cancer[J].Gastroenterology, 2010, 139(3):1041-1051.doi: 10.1053/j.gastro.2010.05.084.
[16] Mizutani Y, Kobayashi H, Iida T, et al.Meflin-Positive Cancer-Associated Fibroblasts Inhibit Pancreatic Carcinogenesis[J].Cancer Res, 2019, 79(20):5367-5381.doi: 10.1158/0008-5472.CAN-19-0454.
[17] Farrow B, Rowley D, Dang T, et al.Characterization of tumorderived pancreatic stellate cells[J].J Surg Res, 2009, 157(1):96-102.doi: 10.1016/j.jss.2009.03.064.
[18] Fujiwara K, Ohuchida K, Mizumoto K, et al.CD271- subpopulation of pancreatic stellate cells correlates with prognosis of pancreatic cancer and is regulated by interaction with cancer cells[J].PLoS One, 2012, 7(12):e52682.doi: 10.1371/journal.pone.0052682.
[19] Tjomsland V, Aasrum M, Christoffersen T, et al.Functional heterogeneity in tumor-derived human pancreatic stellate cells:Differential expression of HGF and implications for mitogenic signaling and migration in pancreatic cancer cells[J].Oncotarget,2017, 8(42):71672-71684.doi: 10.18632/oncotarget.17800.
[20] Öhlund D, Handly-Santana A, Biffi G, et al.Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer[J].J Exp Med, 2017, 214(3):579-596.doi: 10.1084/jem.20162024.
[21] Strobel O, Dadabaeva N, Felix K, et al.Isolation and culture of primary human pancreatic stellate cells that reflect the context of their tissue of origin[J].Langenbecks Arch Surg, 2016, 401(1):89-97.doi: 10.1007/s00423-015-1343-6.
[22] Bynigeri RR, Jakkampudi A, Jangala R, et al.Pancreatic stellate cell: Pandora's box for pancreatic disease biology[J].World J Gastroenterol, 2017, 23(3):382-405.doi: 10.3748/wjg.v23.i3.382.
[23] Neuzillet C,Tijeras-Raballand A, Ragulan C , et al.Inter- and intratumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma[J].J Pathol, 2019,248(1):51-65.doi: 10.1002/path.5224.
[24] Farooqi AA, Desai NN, Qureshi MZ, et al.Exosome biogenesis,bioactivities and functions as new delivery systems of natural compounds[J].Biotechnol Adv, 2018, 36(1):328-334.doi: 10.1016/j.biotechadv.2017.12.010.
[25] Street JM, Koritzinsky EH, Glispie DM, et al.Urine Exosomes: An Emerging Trove of Biomarkers[J].Adv Clin Chem, 2017, 78:103-122.doi: 10.1016/bs.acc.2016.07.003.
[26] Khalyfa A, Gozal D, Chan WC, et al.Circulating plasma exosomes in obstructive sleep apnoea and reverse dipping blood pressure[J].Eur Respir J, 2020, 55(1), pii: 1901072.doi:10.1183/13993003.01072-2019.
[27] Li K, Chen Y, Li A, et al.Exosomes play roles in sequential processes of tumor metastasis[J].Int J Cancer, 2019, 144(7):1486-1495.doi: 10.1002/ijc.31774.
[28] Liao W, Du Y, Zhang C, et al.Exosomes: The next generation of endogenous nanomaterials for advanced drug delivery and therapy[J].Acta Biomater, 2019, 86:1-14.doi: 10.1016/j.actbio.2018.12.045.
[29] Yang TT, Liu CG, Gao SC, et al.The Serum Exosome Derived MicroRNA-135a, -193b, and -384 Were Potential Alzheimer's Disease Biomarkers[J].Biomed Environ Sci, 2018, 31(2):87-96.doi: 10.3967/bes2018.011.
[30] Hu C, Chen M, Jiang R, et al.Exosome-related tumor microenvironment[J].J Cancer, 2018, 9(17):3084-3092.doi:10.7150/jca.26422.
[31] Wang B, Wang ZM, Ji JL, et al.Macrophage-Derived Exosomal Mir-155 Regulating Cardiomyocyte Pyroptosis and Hypertrophy in Uremic Cardiomyopathy[J].JACC Basic Transl Sci, 2020,5(2):148-166.doi: 10.1016/j.jacbts.2019.10.011.
[32] Aguilera-Rojas M, Sharbati S, Stein T, et al.Deregulation of miR-27a may contribute to canine fibroblast activation after co-culture with a mast cell tumour cell line[J].FEBS Open Bio, 2020, doi:10.1002/2211-5463.12831.[Epub ahead of print]
[33] Gao Q, Lei F, Zeng Q, et al.Functional Passenger-Strand miRNAs in Exosomes Derived from Human Colon Cancer Cells and Their Heterogeneous Paracrine Effects[J].Int J Biol Sci, 2020,16(6):1044-1058.doi: 10.7150/ijbs.40787.
[34] Melo SA, Sugimoto H, O'Connell JT, et al.Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis[J].Cancer Cell, 2014, 26(5):707-721.doi: 10.1016/j.ccell.2014.09.005.
[35] Li C, Li X, Zhao B, et al.Exosomes derived from miR-544-modified mesenchymal stem cells promote recovery after spinal cord injury[J].Arch Physiol Biochem, 2020, 6:1-7.doi:10.1080/13813455.2019.1691601.
[36] Takikawa T, Masamune A, Yoshida N, et al.Exosomes Derived From Pancreatic Stellate Cells: MicroRNA Signature and Effects on Pancreatic Cancer Cells[J].Pancreas, 2017, 46(1):19-27.doi:10.1097/MPA.0000000000000722.
[37] Leca J, Martinez S, Lac S, et al.Cancer-associated fibroblastderived annexin A6+ extracellular vesicles support pancreatic cancer aggressiveness[J].J Clin Invest, 2016, 126(11):4140-4156.doi: 10.1172/JCI87734.
[38] Zhao H, Yang L, Baddour J, et al.Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism[J].Elife,2016, 5:e10250.doi: 10.7554/eLife.10250.
[39] Richards KE, Zeleniak AE, Fishel ML, et al.Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells[J].Oncogene, 2017, 36(13):1770-1778.doi: 10.1038/onc.2016.353.
[40] Dutta S, Lai A, Scholz-Romero K, et al.Hypoxia-induced small extracellular vesicle proteins regulate proinflammatory cytokines and systemic blood pressure in pregnant rats[J].Clin Sci (Lond),2020, pii: CS20191155.doi: 10.1042/CS20191155.[Epub ahead of print]
[41] Masamune A, Yoshida N, Hamada S, et al.Exosomes derived from pancreatic cancer cells induce activation and profibrogenic activities in pancreatic stellate cells[J].Biochem Biophys Res Commun,2018, 495(1):71-77.doi: 10.1016/j.bbrc.2017.10.141.
[42] Zhang YF, Zhou YZ, Zhang B, et al.Pancreatic cancer-derived exosomes promoted pancreatic stellate cells recruitment by pancreatic cancer[J].J Cancer, 2019, 10(18):4397-4407.doi:10.7150/jca.27590.
[43] Nielsen N, Kondratska K, Ruck T, et al.TRPC6 channels modulate the response of pancreatic stellate cells to hypoxia[J].Pflugers Arch,2017, 469(12):1567-1577.doi: 10.1007/s00424-017-2057-0.
[44] Storck H, Hild B, Schimmelpfennig S, et al.Ion channels in control of pancreatic stellate cell migration[J].Oncotarget, 2017, 8(1):769-784.doi: 10.18632/ oncotarget.13647.
[45] Zhao W, Ajani JA, Sushovan G, et al.Galectin-3 Mediates Tumor Cell-Stroma Interactions by Activating Pancreatic Stellate Cells to Produce Cytokines via Integrin Signaling[J].Gastroenterology,2018, 154(5):1524-1537.doi: 10.1053/j.gastro.2017.12.014.
[46] 黄昊苏, 严璐, 龙稹朴, 等.肿块型慢性胰腺炎的临床特征及诊治: 附16例报告[J].中国普通外科杂志, 2019, 28(3):320-326.doi:10.7659/j.issn.1005-6947.2019.03.011.Huang HS, Yan L, Long ZP, et al.Clinical features of mass-forming chronic pancreatitis and its diagnosis and treatment: a report of 16 cases[J].Chinese Journal of General Surgery, 2019, 28(3):320-326.doi:10.7659/ j.issn.1005-6947.2019.03.011.
[47] 刘景鸿, 施小六.遗传性胰腺炎发病机制及治疗研究进展[J].中国普通外科杂志, 2019, 28(3):335-342.doi:10.7659/j.issn.1005-6947.2019.03.013.Liu JH, Shi XL.Research progress of pathogenesis and treatment of hereditary pancreatitis[J].Chinese Journal of General Surgery,2019, 28(3):335-342.doi:10.7659/j.issn.1005-6947.2019.03.013.
[48] Ramakrishnan P, Loh WM, Gopinath SCB, et al.Selective phytochemicals targeting pancreatic stellate cells as new anti-fibrotic agents for chronic pancreatitis and pancreatic cancer[J].Acta Pharm Sin B, 2020, 10(3):399-413.doi: 10.1016/j.apsb.2019.11.008.doi:10.1016/j.apsb.2019.11.008.
[49] Charrier A, Chen R, Chen L, et al.Connective tissue growth factor(CCN2) and microRNA-21 are components of a positive feedback loop in pancreatic stellate cells (PSC) during chronic pancreatitis and are exported in PSC-derived exosomes[J].J Cell Commun Signal, 2014, 8(2):147-156.doi: 10.1007/s12079-014-0220-3.
[50] Huang CF, Sun CC, Zhao F,et al.miR-33a levels in hepatic and serum after chronic HBV-induced fibrosis[J].J Gastroenterol, 2015,50(4):480-490.doi: 10.1007/s00535-014-0986-3.
[51] Zhou G, Lin W, Fang P,et al.MiR-10a improves hepatic fibrosis by regulating the TGFβl/Smads signal transduction pathway[J].Exp Ther Med, 2016, 12(3):1719-1722.doi: 10.3892/etm.2016.3542.
[52] Liu XY, He YJ, Yang QH, et al.Induction of autophagy and apoptosis by miR-148a through the sonic hedgehog signaling pathway in hepatic stellate cells[J].Am J Cancer Res, 2015,5(9):2569-2589.
[53] Wang H, Jiang Y, Lu M, et al.STX12 lncRNA/miR-148a/SMAD5 participate in the regulation of pancreatic stellate cell activation through a mechanism involving competing endogenous RNA[J].Pancreatology, 2017, 17(2):237-246.doi: 10.1016/j.pan.2017.01.010.
[54] Suzuki R, Asama H, Waragai Y, et al.Fibrosis-related miRNAs as serum biomarkers for pancreatic ductal adenocarcinoma[J].Oncotarget, 2017, 9(4):4451-4460.doi: 10.18632/oncotarget.23377.
[55] Yan B, Cheng L, Jiang Z, et al.Resveratrol Inhibits ROSPromoted Activation and Glycolysis of Pancreatic Stellate Cells via Suppression of miR-21[J].Oxid Med Cell Longev, 2018,2018:1346958.doi: 10.1155/2018/1346958.
[56] Xue R, Wang J, Yang L, et al.Coenzyme Q10 Ameliorates Pancreatic Fibrosis via the ROS-Triggered mTOR Signaling Pathway[J].Oxid Med Cell Longev, 2019, 2019:8039694.doi:10.1155/2019/8039694.
[57] Xue R, Yang J, Wu J, et al.Coenzyme Q10 inhibits the activation of pancreatic stellate cells through PI3K/AKT/mTOR signaling pathway[J].Oncotarget, 2017, 8(54):92300-92311.doi: 10.18632/oncotarget.21247.
[58] Xu XF, Liu F, Xin JQ, et al.Respective roles of the mitogenactivated protein kinase (MAPK) family members in pancreatic stellate cell activation induced by transforming growth factor-β1(TGF-β1)[J].Biochem Biophys Res Commun, 2018, 501(2):365-373.doi: 10.1016/j.bbrc.2018.04.176.
[59] Wu L, Zhang Q, Mo W, et al.Quercetin prevents hepatic fibrosis by inhibiting hepatic stellate cell activation and reducing autophagy via the TGF-β1/Smads and PI3K/Akt pathways[J].Sci Rep, 2017,7(1):9289.doi: 10.1038/s41598-017-09673-5.
[60] Sun L, Qu L, Brigstock DR, et al.Biological and Proteomic Characteristics of an Immortalized Human Pancreatic Stellate Cell Line[J].Int J Med Sci, 2020, 17(1):137-144.doi: 10.7150/ijms.36337.
