结肠癌是中老年人的常见消化系统恶性肿瘤,其发病率和致死率较高。因此,其诊断与治疗在国内外引发了高度关注[1]。结肠癌确诊后建议早期行根治术,近年来随着微创技术的发展,腹腔镜结肠切除术已被广泛用于结肠癌根治术中[2-3]。目前,对于腹腔镜结肠切除术的入路选择仍存在较大争议。腹腔镜结肠切除术的关键在于选取合适的手术入路,目前临床使用的常用入路包括中间入路、侧方入路及在侧方入路基础上改善的尾侧入路等[2]。既往研究常选择中间入路作为腹腔镜结肠根治术的标准入路,基本符合肿瘤学根治原则,但其对术者水平的要求较高,当患者出现肠系膜水肿等状况时可加大操作难度[4]。近年来有学者[5]提倡采用肠系膜根部右髂窝附着处为手术入路,即尾侧入路,具有可操作性强、安全性好的优势。
由于右半结肠区域血管走行复杂,因此右半结肠切除术的操作难度更高,需要慎重选取手术入路。我院对收治的右侧结肠癌患者进行腹腔镜右半结肠切除术的治疗,采用尾侧入路与中间入路的对比研究,旨在探寻腹腔镜结肠切除术的最佳手术入路,现报告如下。
1 资料与方法
1.1 一般资料
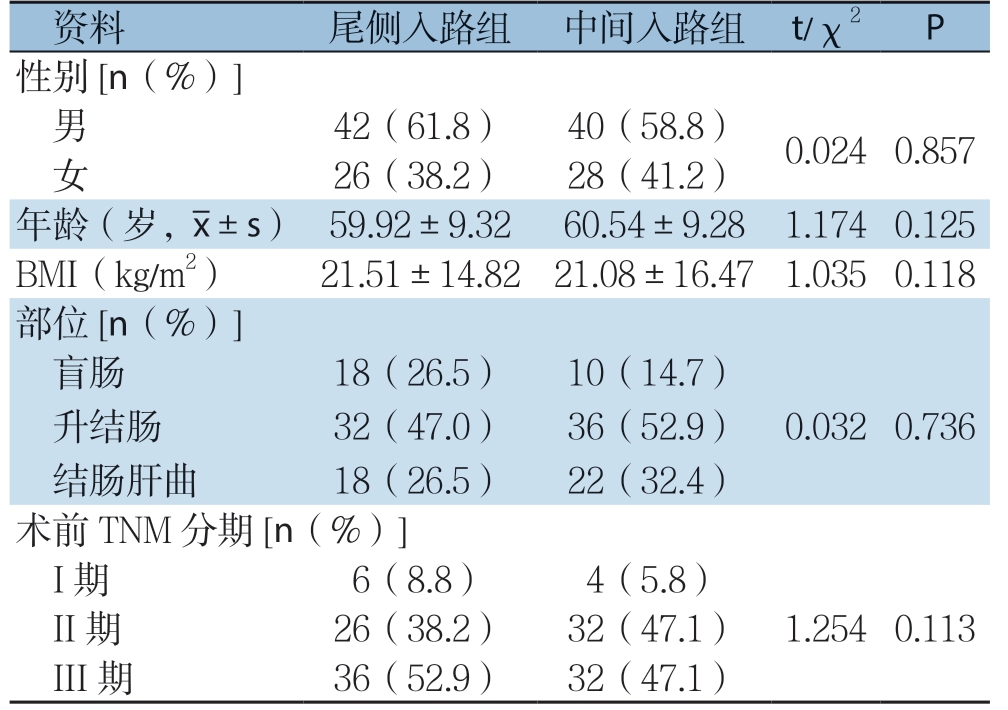
研究对象均为我院2016年5月—2019年5月收治的确诊为右半结肠癌的患者,从中选取符合研究需求者136例。纳入标准:⑴ 经病理学确诊为右半结肠癌;⑵ 癌症TNM分期在I~III期;⑶ 美国麻醉医师协会(A m e r i c a n S o c i e t y o f Anesthesiologists,ASA)分级≤3级;⑷ 随访时间≥2 4 个月;⑸ 所有患者均进行术前沟通,根据自身接受程度与经济能力自愿选择手术入路,并签署知情同意书。排除标准:⑴ 存在其他原因引起的结肠炎症或其他系统肿瘤;⑵ 既往接受过其余结肠癌相关手术治疗或新辅助化疗;⑶ 肿瘤转移至邻近脏器或合并远处转移;⑷ 存在心、肺等严重器官功能衰竭的症状。患者均接受腹腔镜右半结肠切除术,依据入路选择的不同、利用随机数表法分为两组,尾侧入路组68例采用尾侧入路,中间入路组68例采用中间入路。患者中男82例,女54例,年龄47~69岁,平均(60.04±9.29)岁。对比分析两组患者的性别、平均年龄、体质量指数(body mass index,BMI)、部位、术前TNM分期等一般资料比较,差异均无统计学意义(均P>0.05)(表1)。本研究已通过医院伦理委员会批准备案。
表1 两组患者术前基线资料比较(n=68)
Table 1 Comparison of preoperative baseline data between the two groups of patients (n=68)
?
1.2 手术方法
所有患者接受相同的手术人员进行手术,均接受相同的全麻与气管插管,采取5孔法进行操作(图1A)。充分评估腹腔中肿瘤病灶的分布与血液供应状况,完成术前准备。尾侧入路:⑴ 肠系膜上血管的游离(图1B):显露小肠系膜与后腹膜间的相交线,定位入Toldt's间隙(右结肠后间隙),由尾侧至头侧继续显露右侧结肠邻近的区域并清扫淋巴结,显露区域:左至肠系膜上血管左缘,右至会阴区血管右缘,上至胃与十二指肠(图1C);⑵ 肠系膜上血管的层次解剖与淋巴结清扫:以肠系膜上血管的腹膜投影为起点,对肠系膜上血管进行分层解剖,并与前述游离的Toldt's间隙进行连结(图1 D-E)。由尾侧至头侧沿肠系膜上血管封闭邻近血管,并处理对应的淋巴结(图1F-G);⑶ 右半结肠外侧区域的游离与淋巴结清扫:于横结肠上部游离胃大弯处胃结肠韧带,对横结肠后间隙进行分层解剖并连结Toldt's间隙,继而游离肝结肠韧带等,充分游离右半结肠,并处理对应的淋巴结(图1H)。选取右下腹切口,进行横结肠与回肠的交联吻合操作。将肠管放回腹腔,闭合肠系膜裂孔,常规冲洗清洁并关腹,监测术后状态(图1I)。中间入路:选取回结肠血管下缘作为手术起点进行肠系膜的切开,定位入Toldt's间隙,顺沿肠系膜上血管的走行方向游离右半结肠,离断右半结肠血管并清扫对应淋巴结。横结肠上区血管的处置同上述。选取上腹正中切口,进行横结肠与回肠的交联吻合操作。将肠管放回腹腔,闭合肠系膜裂孔,常规冲洗清洁并关腹。
1.3 观察指标
⑴ 术中一般指标:两组患者手术时间、术中出血量、因腹腔镜困难而中转开腹率等;⑵ 术后一般指标:两组患者术后通气时间、通便时间、恢复饮食时间、引流量、引流时间、住院时间等指标;⑶ 术后病理指标:两组患者术后淋巴结清扫数及TNM分期情况;⑷ 术后并发症:两组患者切口感染、肺部炎症、吻合口瘘及肠梗阻等术后并发症发生率;⑸ 远期预后:比较两组患者术后 2年存活率,利用Kaplan-Meier法进行生存分析并绘制生存曲线。
1.4 统计学处理
采用SPSS 26.0 软件进行统计分析,计数资料采用例数(百分率)率[n(%)]表示,两组间比较采用Pearson χ2检验 ,理论频数或样本量不达标时使用连续性校正的χ2检验;计量资料采用均数±标准差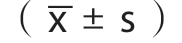 表示,两组间比较采用 t检验,以P<0.05作为结果显著差异的指标。利用Kaplan-Meier法进行生存分析并绘制生存曲线。
表示,两组间比较采用 t检验,以P<0.05作为结果显著差异的指标。利用Kaplan-Meier法进行生存分析并绘制生存曲线。
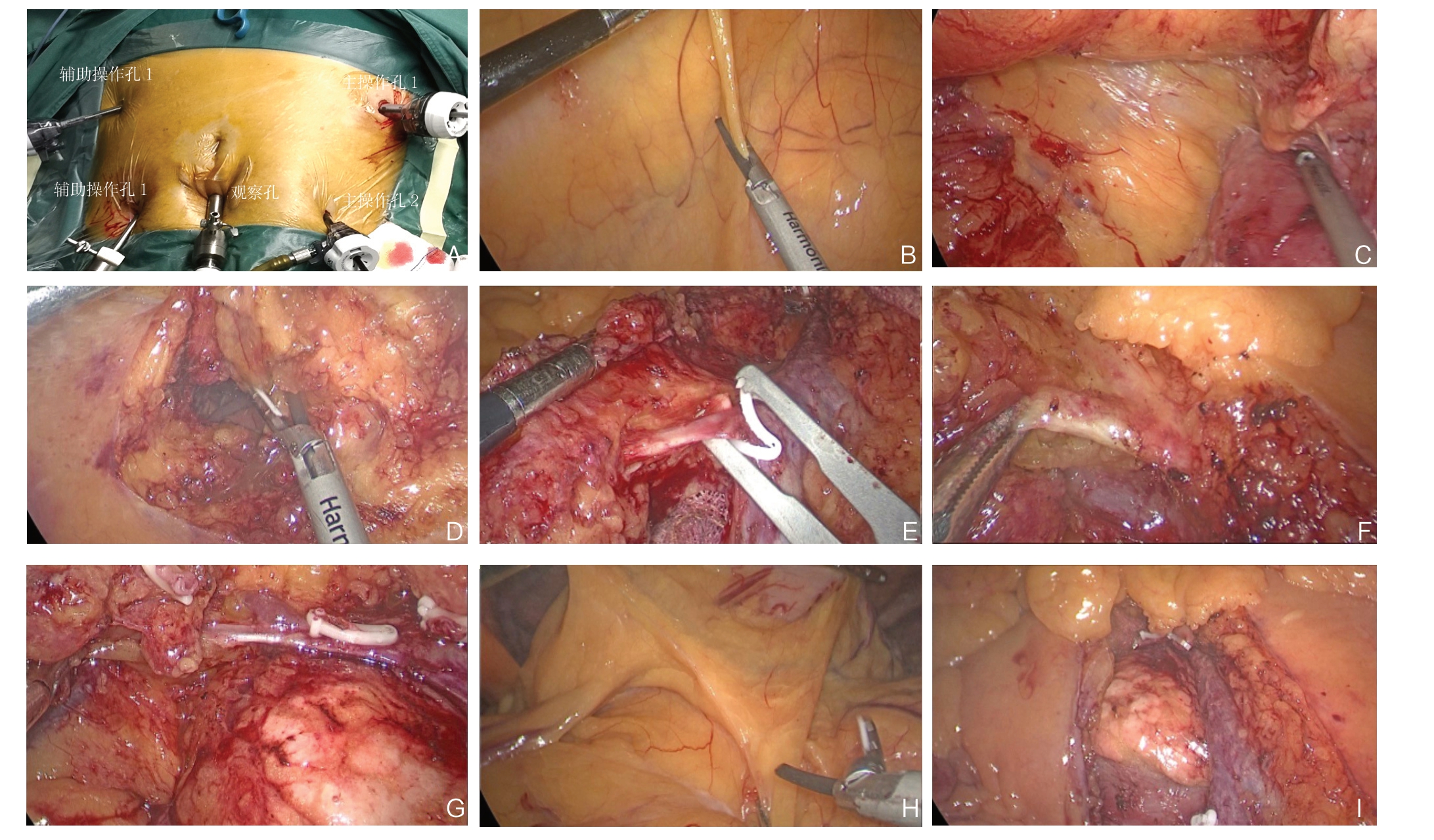
图1 术中图片 A:Trocar 布局;B:尾侧入路切开肠系膜膜桥;C:手术场景腹膜后展示;D:切开膜桥与腹膜后间隙连结;E:离断回结肠血管;F:解剖中结肠动脉左右支;G:解剖胃结肠干;H:血管弓外离断胃结肠韧带;I:手术后状态
Figure 1 Intraoperative views A: Trocar arrangement; B: Incision of the mesenteric bridge by caudal-to-cranial approach; C: Posterior peritoneal display of the surgical scene; D: Incision of the connection of the mesenteric bridge to the retroperitoneal space; E: Severing the colonic vessels; F: Dissection of the left and right branches of the middle colic artery; G: Dissection of the gastrocolic trunk; H: Division of the gastric colonic ligament outside the vascular arch; I: Postoperative state
2 结 果
2.1 两组患者术中情况
两组手术均顺利完成。尾侧入路组的手术时间、术中出血量、中转开腹率等指标均明显低于中间入路组(均P<0.05)(表2)。
2.2 两组患者术后情况
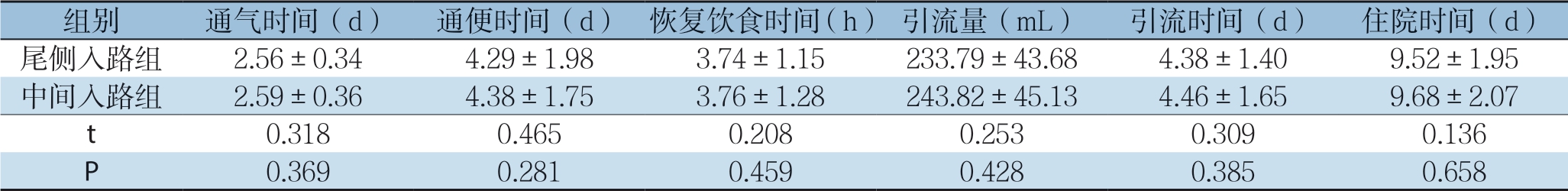
两组患者的通气时间、通便时间、恢复饮食时间、引流量、引流时间、住院时间等指标差异无统计学意义(均P>0.05)(表3)。
表2 两组患者术中指标比较(n=68)
Table 2 Comparison of intraoperative variables between the two groups of patients (n=68)

表3 两组患者术后指标的比较(n=68
Table 3 Comparison of postoperative indexes between two groups of patients (n=68
2.3 两组患者术后病理结果
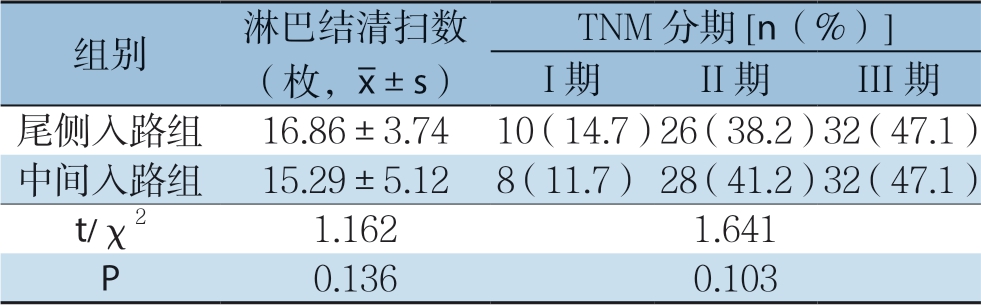
两组患者术后淋巴结清扫数、TNM分期等病理指标差异无统计学意义(均P>0.05)(表4)。
表4 两组患者术后病理指标比较(n=68)
Table 4 Comparison of postoperative pathological results between two groups of patients (n=68)
2.4 两组患者术后并发症指标情况
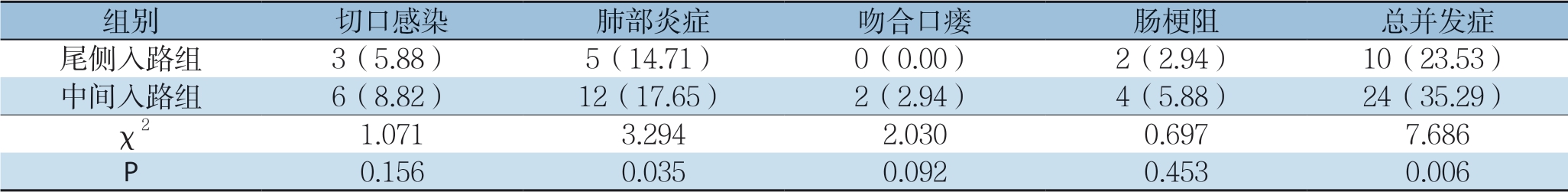
尾侧入路组患者的肺部炎症及总并发症发生率均明显低于中间入路组(均P<0.05)。并发症予以保守对症处置后均有好转,无因严重并发症而终止研究的病例(表5)。
表5 两组患者术后并发症比较[n=68,n(%)]
Table 5 Comparison of postoperative complications between two groups of patients [n=68, n (%)]
2.5 两组患者预后情况
所有患者均通过电话、门诊等方式完成随访,随访终止于术后2 4 个月或死亡终止。尾侧入路组2 年生存率为8 2.3 5%,中间入路组为52.94%,尾侧入路组患者的2年生存率明显高于中间入路组(χ2=13.439,P<0.05)。两组患者2年内的生存曲线见图2。
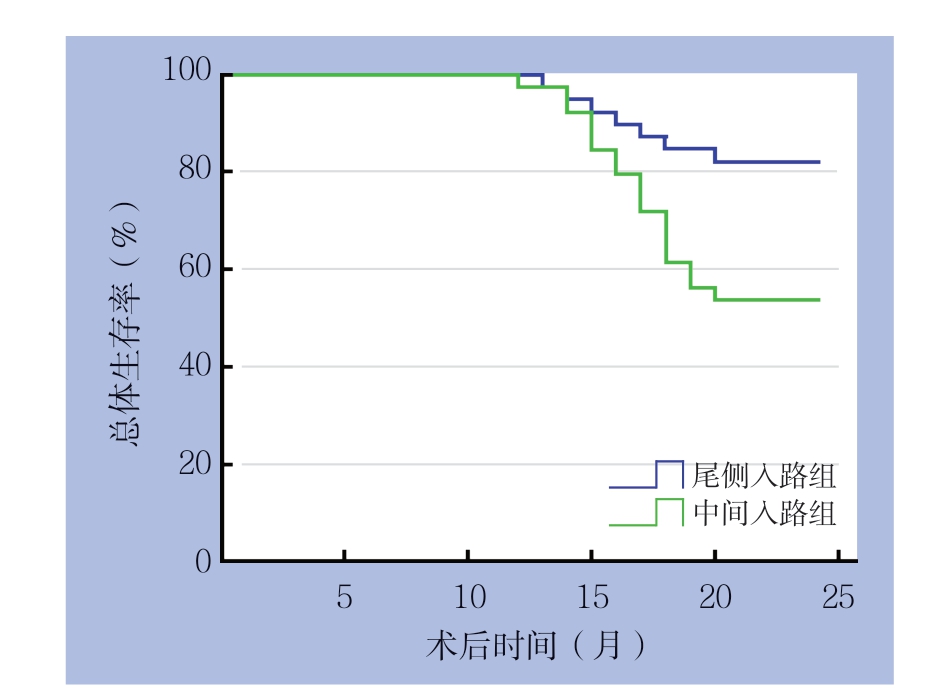
图2 两组患者术后生存曲线
Figure 2 Survival curves of the two groups of patients after operation
3 讨 论
3.1 腹腔镜下结肠癌根治术的现状分析
结肠癌是我国中老年人群中高发的消化系统恶性肿瘤。右半结肠癌的临床表现严重、预后较差,确诊后应在适应征允许的情况下尽早接受结肠癌根治术[6-7]。近年来,腹腔镜手术已逐渐取代开放手术成为结肠癌根治术的主流术式,具有直观、微创的优势[8]。腹腔镜手术的重点在于选择合适的手术入路,有利于操作者准确发现手术层次并分离,降低手术时间和手术创伤,提高术后恢复效果和预后质量;但目前尚无国际通用的结肠癌根治术的入路指导原则[9]。腹腔镜右半结肠切除术的传统入路包括中间入路和侧方入路,均具有较大的应用劣势。
3.2 传统入路的操作要点及优劣分析
肿瘤根治操作中的重点在于遵循“no-touch”原则,即处理肿瘤病灶时尽量不直接接触,规避因操作失误而引发医源性肿瘤扩散[10]。中间入路的操作要点在于先封闭肿瘤的供血血管、游离右半结肠系膜,后离断肠系膜及肠管[11]。中间入路的优势在于可保证操作视野的充足与肿瘤血管处理的彻底性,预防腹腔中残存肿瘤组织,可充分体现“no-touch”原则[12]。中间入路术中应维持肠系膜受力状态的稳定性,有利于精确定位手术层面并保证操作区域。但中间入路的劣势在于结肠解剖结构与操作步骤的复杂性,手术的效果受肠系膜厚度的影响较大,若肠系膜过厚易导致术中致伤肠系膜和肠管,若肠系膜过厚则无法准确定位血管走行[13]。因此,中间入路对于肠系膜厚度的要求较高,且手术难度较大,具有较高的操作门槛、难以进行充分推广。
侧方入路的操作步骤类似于开放手术,首先定位小肠系膜根部并离断小肠系膜,游离右半结肠,再结扎肿瘤区域走行的血管,最后进行结肠的切除吻合[14]。因此,相较于中间入路,侧方入路有利于简化手术步骤,对术者操作条件与技术水平的要求较低[15]。此外,侧方入路的通用性更广,即使根治指征不明确的患者也可以选用,有利于准确判定肿瘤附近血液供应情况。Munkedal等16]表明,相较于中间入路,侧方入路可有效降低手术创伤,具有更少的出血量与手术时间,在保证肿瘤切除范围的同时减少对机体免疫系统的干扰。但侧方入路的缺陷在于肿瘤细胞直接受压后存在转移播散的风险,不符合“no-touch”原则;且侧方入路对于改善肿瘤预后的远期效果尚未明确[17]。此外,本术式虽然操作的可行性好,但术中需变换体位,影响手术过程的顺畅进行。
3.3 尾侧入路的操作要点及优势分析
传统中间入路与侧方入路均可完成手术目的,取得一定的治疗效果,但两种入路方式均存在较大劣势,需要进行完善。本次研究采用基于侧方入路改善后的尾侧入路法,应用于腹腔镜右半结肠切除术。手术步骤包括肠系膜上血管的游离、肠系膜上血管的解剖及对应淋巴结的清扫、右半结肠外侧区域的游离等。手术起点选取于肠系膜与后腹膜的相交线,有利于快速定位肠系膜间隙,并保证充足的操作视野[18]。手术采用直观离断操作,可保证离断层次的准确性并规避医源性损伤;暴露右结肠间隙后可较好地与后方间隙相通,对于全段血管的显示效果较好,有利于进行血源性根治[19]。因尾侧入路简便、安全、微创的优势,近年来已被国内外临床研究广泛采用。
尾侧入路的优势分析如下[20-22]:⑴ 尾侧入路以侧方入路为基础,同样具有流程简单、可行性好、手术创伤小等优势,可有效降低手术时间、术中出血量等术中指标;⑵ 尾侧入路较好地改善了侧方入路的劣势,术中体位固定,规避了频繁更换体位的情况,确保了手术过程的连续性与顺畅性;⑶ 尾侧入路通过改善肠系膜游离的方式,避免术中接触并挤压肿瘤组织,预防肿瘤组织在腹腔内播散,符合“no-touch”原则。
3.4 尾侧入路应用于腹腔镜下结肠癌根治术的结果分析
本次研究中,对两组患者术中、术后一般指标及并发症指标进行对比,结果表明:尾侧入路组患者的手术时间、术中出血量、中转开腹率等指标均明显低于中间入路组,分析原因在于尾侧入路有利于高效、精确定位至需要的解剖层次,降低了手术的操作步骤及创伤,因此可有效控制手术时间与术中出血量。中间入路采用首先离断肿瘤血供的操作,易损伤正常血管;而尾侧入路的术创伤发生率低,因此中转开腹率更低。两组患者的通气时间、通便时间、恢复饮食时间、引流量、引流时间、住院时间等指标的比较均差异较小,无统计学意义,提示两种入路患者可取得一致的术后恢复效果。尾侧入路组患者的术后总并发症的发生率低于中间入路组,分析原因在于尾侧入路充分体现了微创理念与“no-touch”原则,优化了操作步骤,手术创伤较低,因而降低了术后并发症的发生率[23]。
本次研究中,对比两组患者术后病理及预后的结果表明:两组患者术后淋巴结清扫数、TNM分期等病理指标差异无统计学意义,尾侧入路组患者的2年生存率为82.35%(56/68),中间入路组患者的2年生存率为52.94%(36/68),尾侧入路组患者的2年生存率明显高于中间入路组,原因在于两组患者手术过程中均依据根治要求进行肿瘤病灶的清除,均可达到满意的临床效果。针对手术层面进行分析,尾侧入路可保证手术层面局限于肠系膜区域,可把握准确的解剖层次,保证手术的临床疗效与预后水平[24-25]。近年来,随着微创理念的不断深入,尾侧入路的可行性逐渐提出并得到临床印证。Du等[2]在腹腔镜右半结肠癌的根治中采用尾侧入路,结果表明手术时间、术中出血量明显降低,术后不良反应发生率控制在10%以内,淋巴结清扫35枚、阳性5枚,其结果提示尾侧入路具有操作简便、安全性好的特点,符合肿瘤根治原则。Toyoda等[26]研究结果表明,尾侧入路法可有效缩短临床医师的学习曲线,利于向基层医院推广。有学者[27-28]建议在既往的尾侧入路上行进一步改良,可进一步缩短围手术期指标、提高治疗安全性。
3.5 本次研究的局限性及改进
本次研究表明,在腹腔镜右半结肠切除术中采用尾侧入路具有安全、微创、简便、可操作性好的优势,但本次研究仍存在部分有待完善的问题:⑴ 本研究范围仅局限于中间入路与尾侧入路,有待于后续探讨其他入路或联合入路的治疗效果;如Huma等[29]尝试采用改良右半肠切除术、3D高清腹腔镜等方式进行辅助手术,可能对提高手术操作精度、拓展手术视野具有重要效果,有待于后续学者的进一步研究;⑵ 本次研究样本量一般,且研究时程有限。有待于后续继续改进手术方法,行进一步长时程、大样本量研究。
综上所述,相较于中间入路,在腹腔镜右半结肠切除术中采用尾侧入路具有安全、微创、简便、可操作性好的优势,有利于保证手术视野的充分及解剖定位的准确,可在保证相似的临床治疗效果的同时,较好地减少手术时间、术中出血量、中转开腹率等指标,并降低术后并发症的发生率、改善患者预后。建议行进一步大样本量研究及临床推广。
[1]韦振轩, 陈小勋. X线辅助内镜置入支架联合腹腔镜手术治疗结直肠癌并梗阻的疗效分析[J]. 中国普通外科杂志, 2016, 25(4):475-480. doi:10.3978/j.issn.1005-6947.2016.04.003. Wei ZX, Chen XX. Efficacy analysis of X-ray-assisted endoscopic stent placement and laparoscopic resection in treatment of obstructive colorectal cancer[J]. Chinese Journal of General Surgery, 2016, 25(4):475-480. doi:10.3978/j.issn.1005-6947.2016.04.003.
[2]Du S, Zhang B, Liu Y, et al. A novel and safe approach: middle cranial approach for laparoscopic right hemicolon cancer surgery with complete mesocolic excision[J]. Surg Endosc, 2018, 32(5):2567-2574. doi: 10.1007/s00464-017-5982-2.
[3]张静, 陆晔, 崔新华, 等. 术后早期化疗对III期结直肠癌患者临床预后的影响[J]. 中国普通外科杂志, 2016, 25(4):492-496. doi:10.3978/j.issn.1005-6947.2016.04.006.Zhang J, Lu Y, Cui XH, et al. Impact of early postoperative chemotherapy on the clinical outcomes in patients with stage III colorectal cancer[J]. Chinese Journal of General Surgery, 2016, 25(4):492-496. doi:10.3978/j.issn.1005-6947.2016.04.006.
[4]Chen TC, Liang JT. Robotic Radical Surgery in the Multidisciplinary Approach for the Treatment of Locally Advanced T4 Rectosigmoid Colon Cancer[J]. Dis Colon Rectum, 2019, 62(1):121-122.doi:10.1097/DCR.0000000000001253.
[5]Huang CM, Huang MY, Ma CJ, et al. Neoadjuvant FOLFOX chemotherapy combined with radiotherapy followed by radical resection in patients with locally advanced colon cancer[J]. Radiat Oncol, 2017, 12(1):48. doi:10.1186/s13014-017-0790-3.
[6]Kang SI, Kim DW, Shin E, et al. Clinical Significance of Lymph Node Metastasis in the Mesentery of the Terminal Ileum in Patients With Right-sided Colon Tumors at Different Locations[J]. Dis Colon Rectum, 2018, 61(6):692-697. doi: 10.1097/DCR.0000000000001048.
[7]刘德宝, 孙子雯, 李岩, 等. 直肠癌患者术后化疗联合CIK免疫治疗的临床疗效[J]. 中国普通外科杂志, 2016, 25(8):1186-1192. doi:10.3978/j.issn.1005-6947.2016.08.017. Liu DB, Sun ZW, Li Y, et al. Clinical efficacy of postoperative chemotherapy in combination with CIK immunotherapy in rectal cancer patients[J]. Chinese Journal of General Surgery, 22016, 25(8):1186-1192. doi:10.3978/j.issn.1005-6947.2016.08.017.
[8]冯波, 严夏霖, 张森, 等. 腹腔镜右半结肠癌根治术Henle干的解剖技巧[J]. 中华胃肠外科杂志, 2017, 20(6):635-638. doi:10.3760/cma.j.issn.1671-0274.2017.06.009. Feng B, Yan XL, Zhang S, et al. Anatomical strategies of Henle trunk in laparoscopic right hemi-colectomy for right colon cancer [J]. Chinese Journal of Gastrointestinal Surgery, 2017, 20(6):635-638. doi:10.3760/cma.j.issn.1671-0274.2017.06.009.
[9]Patel SA, Royce TJ, Barysauskas CM, et al. Surveillance Imaging Patterns and Outcomes Following Radiation Therapy and Radical Resection for Localized Extremity and Trunk Soft Tissue Sarcoma[J]. Ann Surg Oncol, 2017, 24(6):1588-1595. doi:10.1245/s10434-016-5755-5.
[10]Uematsu D, Akiyama G, Sugihara T, et al. Transanal Total Pelvic Exenteration With Sphincter-Preserving Surgery[J]. Dis Colon Rectum, 2018, 61(5):641. doi: 10.1097/DCR.0000000000001031.
[11]解龙, 樊启明, 郭磊, 等. 机器人辅助与腹腔镜结直肠癌手术疗效比较的Meta分析[J]. 中国普通外科杂志, 2016, 25(10):1412-1419. doi:10.3978/j.issn.1005-6947.2016.10.008. Xie L, Fan QM, Guo L, et al. Robotic-assisted versus laparoscopic colorectal cancer resection: a Meta-analysis[J]. Chinese Journal of General Surgery, 2016, 25(10):1412-1419. doi: 10.3978/j.issn.1005-6947.2016.10.008.
[12]Peng J, Ou Q, Wu X, et al. Expression of voltage-gated sodium channel Nav1.5 in non-metastatic colon cancer and its associations with estrogen receptor (ER)-β expression and clinical outcomes[J]. Chin J Cancer, 2017, 36(1):89. doi: 10.1186/s40880-017-0253-0.
[13]Zhou X, Wang L, Shen W. Laparoscopic surgery as a treatment option for elderly patients with colon cancer[J]. J BUON, 2017, 22(2):424-430.
[14]Yang X, Wu Q, Jin C, et al. A novel hand-assisted laparoscopic versus conventional laparoscopic right hemicolectomy for right colon cancer: study protocol for a randomized controlled trial[J]. Trials, 2017, 18(1):355. doi: 10.1186/s13063-017-2084-3.
[15]Tsukamoto R, Sugimoto K, Kawano S, et al. Abstract 5254: Postoperative adjuvant chemotherapy improves survival in stage II colon cancer? A propensity score matching analysis[J]. Cancer Res, 2017, 77(13 Supplement):5254. doi:10.1158/1538-7445.am2017-5254.
[16]Munkedal DL, West NP, Iversen LH, et al. Implementation of complete mesocolic excision at a university hospital in Denmark: An audit of consecutive, prospectively collected colon cancer specimens[J]. Eur J Surg Oncol, 2014, 40(11):1494-1501. doi: 10.1016/j.ejso.2014.04.004.
[17]Munkedal DLE, Rosenkilde M, Nielsen DT, et al. Radiological and pathological evaluation of the level of arterial division after colon cancer surgery[J]. Colorectal Dis, 2017, 19(7):O238-O245. doi: 10.1111/codi.13756.
[18]赵月鸣, 邹玉凤, 董莹, 等. 母系印迹表达基因3在结直肠癌组织中表达及其与血管生成的关系[J]. 中国普通外科杂志, 2016, 25(10):1450-1454. doi:10.3978/j.issn.1005-6947.2016.10.014. Zhao YM, Zou YF, Dong Y, et al. Expression of maternally expressed gene 3 in colorectal cancer tissue and its relation with angiogenesis[J]. Chinese Journal of General Surgery, 2016, 25(10):1450-1454. doi:0.3978/j.issn.1005-6947.2016.10.014.
[19]Yeh YS, Tsai HL, Huang CW, et al. Maintenance tegafur-uracil versus observation following an adjuvant oxaliplatin-based regimen in patients with stage III colon cancer after radical resection: study protocol for a randomized controlled trial[J]. Trials, 2017, 18(1):191. doi:10.1186/s13063-017-1904-9.
[20]Oh BY, Park YA, Huh JW, et al. Prognostic Impact of Tumor-Budding Grade in Stages 1-3 Colon Cancer: A Retrospective Cohort Study[J]. Ann Surg Oncol, 2018, 25(1):204-211. doi: 10.1245/s10434-017-6135-5.
[21]Hu JM, Chou YC, Wu CC, et al. Adjuvant chemotherapy with tegafur/uracil for more than 1 year improves disease-free survival for low-risk Stage II colon cancer[J]. J Chin Med Assoc, 2016, 79(9):477-488. doi:10.1016/j.jcma.2016.04.001.
[22]张海容, 袁维堂, 周全博, 等. 机器人与腹腔镜在中下段直肠癌根治术中的应用效果比较[J]. 中华胃肠外科杂志, 2017, 20(5):540-544. doi:10.3760/cma.j.issn.1671-0274.2017.05.013. Zhang HR, Yuan WT, Zhou QB, et al. Efficacy comparison of robotic and laparoscopic radical surgery in the treatment of middle-low rectal cancer[J]. Chinese Journal of Gastrointestinal Surgery, 2017, 20(5):540-544. doi:10.3760/cma.j.issn.1671-0274.2017.05.013.
[23]Zavaruev AV, Mazurenko AA, Bregadze EY, et al. Radical surgery for locally advanced recurrent sigmoid colon cancer[J]. Khirurgiia (Mosk), 2018, (5):108-109. doi: 10.17116/hirurgia20185108-109.
[24]雷伟琦, 胡谦, 王思远, 等. miR-101在结直肠癌中的研究进展[J]. 中国普通外科杂志, 2016, 25(4):592-597. doi:10.3978/j.issn.1005-6947.2016.04.021. Lei WQ, Hu Q, Wang SY, et al. Role of miR-101 in colorectal cancer: recent advances[J]. Chinese Journal of General Surgery, 2016, 25(4):592-597. doi: 10.3978/j.issn.1005-6947.2016.04.021.
[25]Hodgkinson N, Kruger C A, Abrahamse H. Targeted photodynamic therapy as potential treatment modality for the eradication of colon cancer and colon cancer stem cells[J]. Tumour Biol, 2017, 39(10):101042831773469. doi: 10.1177/1010428317734691.
[26]Toyoda S, Horii K, Okumura S, et al. Sigmoid colon cancer with intussusception prolapsing through the anus treated by elective laparoscopic radical surgery[J]. Nihon Shokakibyo Gakkai Zasshi, 2018, 115(1):87-93. doi:10.11405/nisshoshi.115.87.
[27]Wang YC. Effect of preoperative neoadjuvant chemotherapy on the expression of malignant molecules in colon cancer tissue and the degree of trauma caused by radical operation[J]. Journal of Hainan Medical University, 2017, 23(17):113-116.
[28]Nayfe R, Ascha MS, Ismail M, et al. Gastrocolic Fistula as a Presentation of Concomitant Gastric and Colon Cancer in a Patient with a History of Metastatic Pancreatic Adenocarcinoma[J]. J Gastrointest Cancer, 2017, 48(4):379-381. doi:10.1007/s12029-016-9841-5.
[29]Huma N, Rafiq S, Sameen A, et al. Antioxidant potential of buffalo and cow milk Cheddar cheeses to tackle human colon adenocarcinoma (Caco-2) cells[J]. Asian-Australas J Anim Sci, 2018, 31(2):287-292. doi:10.5713/ajas.17.0031.
