胃癌是消化系统常见的恶性肿瘤之一。近年来,我国胃癌发病率和病死率均处于较高水平[1]。据统计,我国每年新增胃癌病例约68万例,同时,每年新增胃癌死亡病例约50万例[2],而患者的5年生存率也仅为27.4%,甚至低于韩国(57.9%)和日本(54.0%)[3]。研究发现,影响胃癌患者预后的一个关键性因素就是淋巴结转移阳性率[4],患者术后病理标本中淋巴结转移阳性率越高,预示着其5年生存率越低[5];同时,患者有无明显的胃周或远处淋巴结肿大,对患者手术方式的选择[6-7]和化疗方案[8]的选择也有直接影响。此外,如果根治术中清扫淋巴结数目不足,患者还会出现阶段性转移现象[9]。
然而,术前相关检查仅能发现明显肿大淋巴结且淋巴结转移的阳性率通常是由术后的病理诊断所证实的。如果能够在疾病的早期明确其有无淋巴结转移甚至转移程度的话,将在指导疾病的治疗和预测患者预后方面发挥非常积极的作用。本研究收集并分析了胃癌患者的相关临床资料信息,从中寻找出影响胃癌淋巴结转移的相关危险因素,以求为术前预测淋巴结转移阳性率提供一定的方法途径。
1 资料与方法
1.1 研究对象
以我院2014年1月—2015年4月收治的胃癌患者相关资料为研究对象。入选标准:⑴ 胃癌患者经术前胃镜活检及术后病理检查确诊;⑵ 患者成功接受根治性胃癌手术并行D2淋巴结清扫;⑶ 切除的淋巴结由专人按照第14版日本《胃癌处理规约》中淋巴结分组进行分拣,并由我院病理科检查淋巴结是否有转移的迹象;⑷ 术后标本中检出的淋巴结数目超过15枚;⑸ 患者术前未接受任何化学治疗。排除标准:⑴ 患者有术前影像学或术中发现其他器官脏器有转移现象;⑵ 患者有其他肿瘤或炎症性疾病。
1.2 方法
收集患者在办理住院手续时的一般术前信息,如年龄、性别、体质量指数(b o d y m a s s i n d e x,B M I)等。此外,还收集了术前及术后血液和病理检查检验所显示的分化程度、浸润深度、Lauren's分型、肿瘤直径和位置以及肿瘤标志物(如AFP、CEA、CA125和CA19-9)等信息。此外,通过在我院患者信息数据库中进行检索、电话随访以及与当地的社区服务中心联系等的方式对这些患者的预后信息进行跟踪收集。
将患者的相关信息按照下列方式进行分组:年龄信息分为<40岁、40~<60岁,≥60岁3组;根据《中国超重/肥胖医学营养治疗专家共识》(2016年版)[10]所规定的BMI 24~<28 kg/m2为超重,BMI≥28 kg/m2为肥胖的标准将BMI分为不同水平;依据我院实验室和病理科所执行的标准将肿瘤标志物分为阳性和阴性。根据美国危重病杂志(AJCC)出版的第7版TNM分期标准对肿瘤浸润深度和淋巴结转移数进行分组。肿瘤位置描述为胃窦、胃角、大弯侧、小弯侧和贲门。由于一些报告指出肿瘤直径的差异对胃癌的治疗和预后有直接影响[11-12],因此将肿瘤直径分为4组:<1 cm、1~<3cm、3~<5 cm以及≥5 cm。
1.3 统计学处理
通过单因素方差分析明确淋巴结转移相关危险因素,多元Logistic回归分析方法确定与淋巴结转移现象相关的独立危险因素,并采用秩和检验法,确定危险因素与淋巴结转移现象之间的具体关系,用ROC曲线检验其敏感度和特异度。同时,用Kaplan-Meier方法制作生存曲线,采用分层Cox比例风险模型的似然比检验进行单变量和多变量生存分析。P<0.05认为有统计学意义。
2 结 果
2.1 患者的临床信息特征
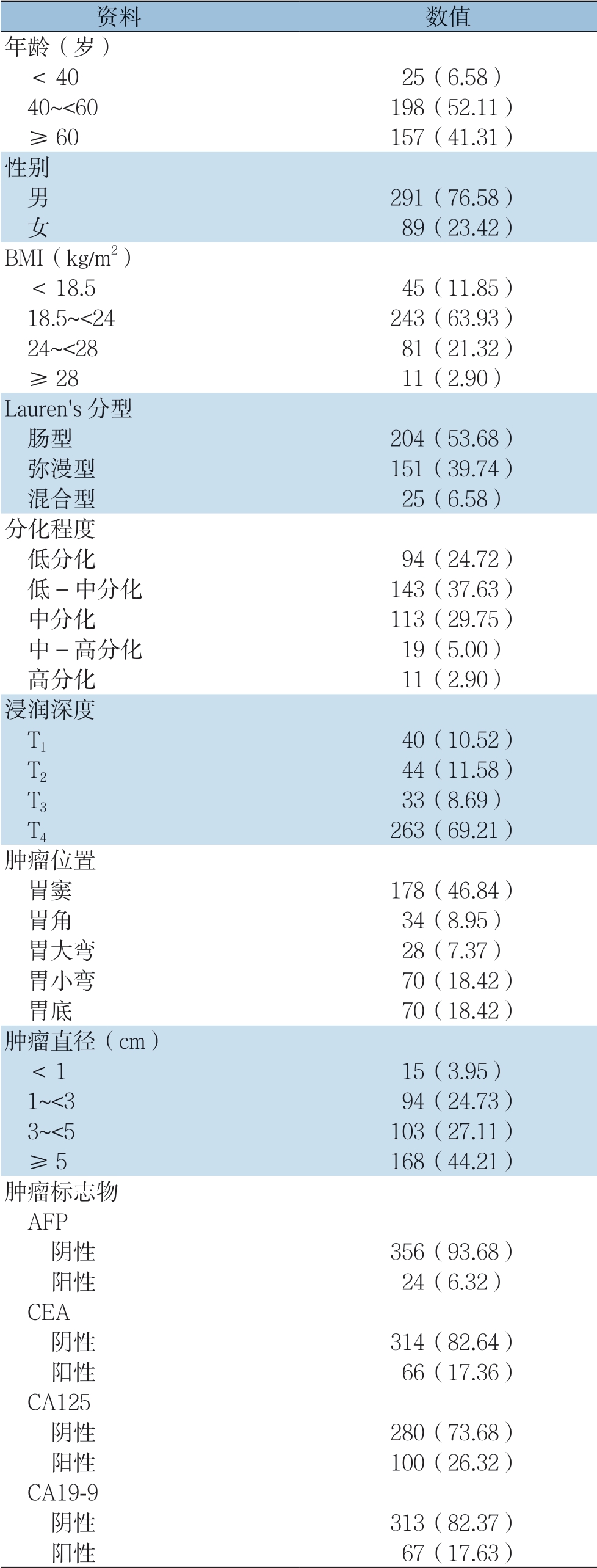
共统计了380例患者资料,并将他们的一般信息资料分组列入表1。在这些患者中,平均年龄为(56.06±10.95)岁,男性占76.58%,女性占23.42%。BMI的平均值为(22.04±3.15)kg/m2,24.22%的患者超重或肥胖(BMI≥24 kg/m2)。肿瘤平均直径(4.47±2.35)cm,肿瘤直径>5 cm者占44.21%。此外,56.38%患者Lauren's分型为肠型,37.63%患者病理显示为中低分化,46.32%患者有肿瘤浸润到浆膜层的现象,46.84%患者肿瘤位于胃窦。大多数患者的肿瘤标志物均为阴性。
表1 380 例胃癌患者的基本资料[n(%)]
Table 1 The general data of the 380 gastric patients [n (%)]
2.2 淋巴结转移现象相关危险因素
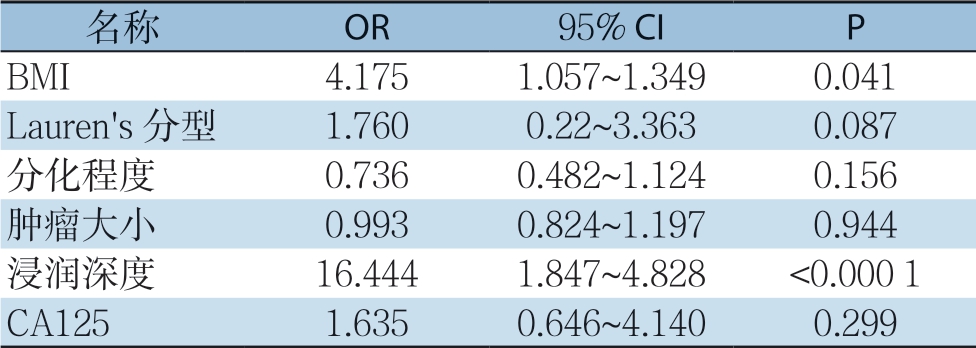
在对淋巴结资料及进行汇总,在所有患者中共检测到1 4 2 0 4 枚淋巴结,其中共2 4 1 例(6 3.4 2%)术后病理显示有淋巴结转移现象。这些患者中,2 1.9 9%的患者淋巴结转移程度达到N1,25.32%达到N2,52.69%达到N3。胃癌淋巴结转移的危险因素单变量分析结果显示,BMI(P=0.006)、Lauren's分型(P=0.002)、浸润深度(P<0.001)、分化程度(P<0.001)、肿瘤直径(P<0.001)、CA125(P=0.017)是影响胃癌淋巴结转移的重要因素(表2)。多因素Logistic回归分析结果显示,仅BMI(OR=4.175,P=0.041)和肿瘤浸润深度(OR=16.444,P<0.000 1)为淋巴结转移现象的独立危险因素(表3)。
表2 淋巴结转移危险因素单因素分析[n(%)]
Table 2 Univariate analysis of factors for lymph node metastasis [n (%)]
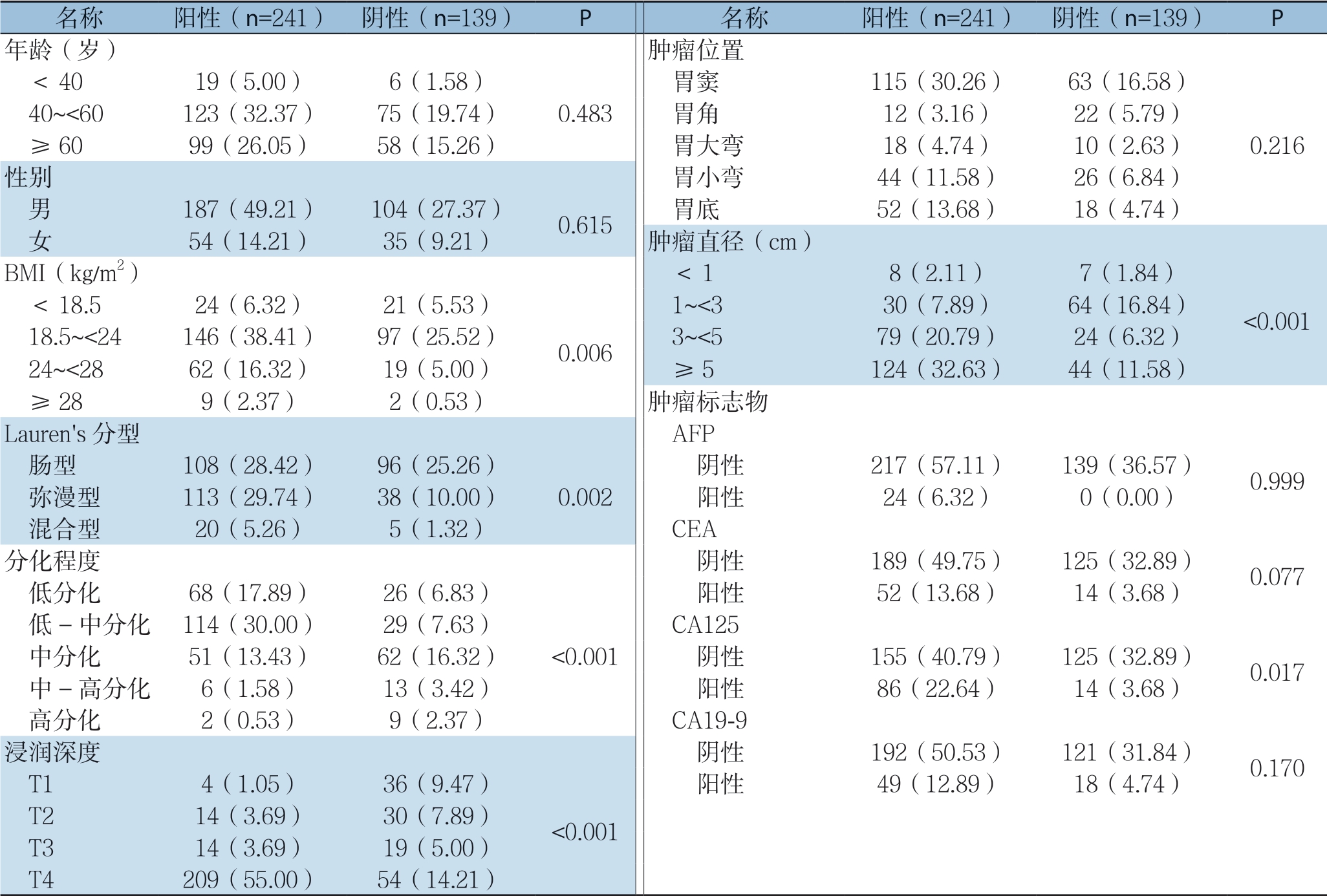
表3 淋巴结转移相关危险因素多因素分析
Table 3 Multivariate analysis of factors for lymph node metastasis
2.3 淋巴结转移现象的预测
进一步研究BMI对淋巴结转移率的影响,相关性分析结果表明:淋巴结转移阳性率与BMI呈正相关(r=1.95,P=0.007)。然后,利用ROC曲线分析B M I 和浸润深度对预测淋巴结转移的敏感度和特异度。结果表明,用BMI≥24 kg/m2 (图1A)对淋巴结转移预测的敏感度为63.16%,特异度为76.84%。同时,用T4的肿瘤浸润深度来预测淋巴结转移的敏感度为63.16%,特异度为53.68%(图1B)。并且,当两个标准结合起来预测淋巴结转移时,其敏感度为63.16%,特异度为88.36%,ROC曲线下面积(AUC)为75.76% (图1C)。

图1 ROC 曲线分析 A:BMI 对淋巴结转移的预测能力;B:浸润深度对淋巴结转移的预测能力;C:BMI 结合浸润深度对胃癌淋巴结转移的预测能力
Figure 1 ROC curve analysis A: Predictive ability of BMI for lymph node metastasis; B: Predictive ability of depth of tumor invasion for lymph node metastasis; C: Predictive ability of BMI combined with depth of tumor invasion for lymph node metastasis
2.4 随访
截止2018年4月30日,患者术后的平均随访时间为18.4个月(0.3~36.9个月),在380例患者中有160例患者死于该疾病,20例患者失访,38例出现复发或其他器官转移,3年总生存率为52.63%。另外,在288例BMI<24 kg/m2的患者中,121例患者死亡,14例失访,26例患者出现复发或其他器官转移;而在BMI≥24 kg/m2的92例患者中39例死亡,6例失访,12例患者出现复发或其他器官转移。Kaplan-Meier分析结果显示,BMI<24 kg/m2的患者3年总生存率(OS)高于BMI≥24 kg/m2的患者,且差异具有统计学意义(53.13% vs. 51.09%,P=0.0457)(图2)。
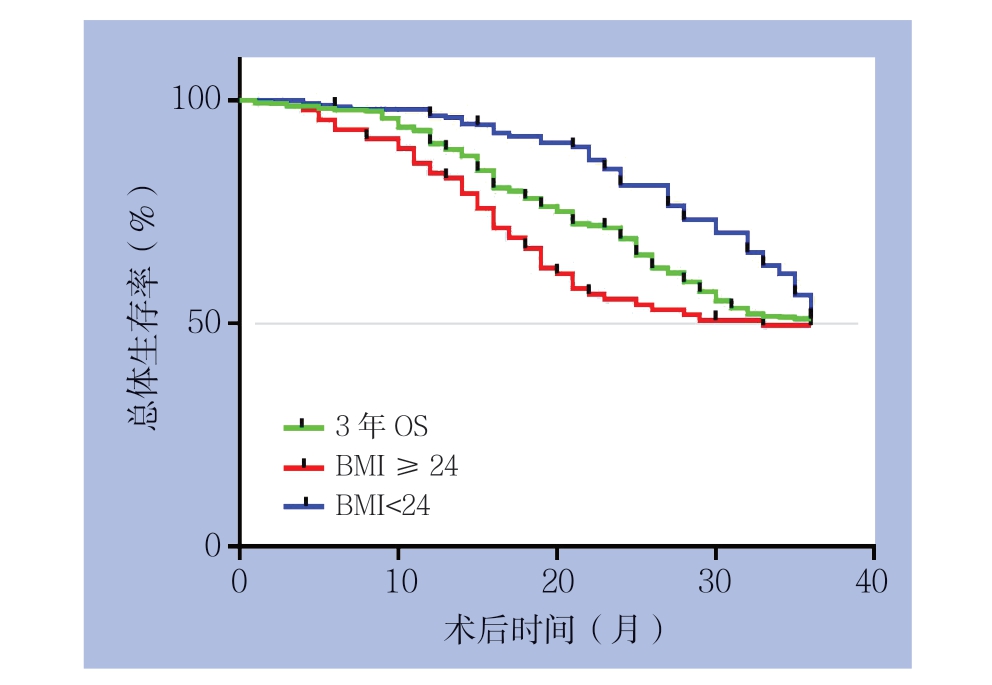
图2 不同BMI 患者生存曲线
Figure 2 Survival curves of patients with different BMI values
3 讨 论
大量研究表明,胃癌患者疾病的组织学类型、淋巴管及静脉的浸润与否、肿瘤的浸润、分化程度、直径以及外周血中的肿瘤标志物水平都是淋巴结转移现象的独立危险因素且和预后之间存在密切关联[13-14]。本研究发现BMI、肿瘤浸润深度、分化程度、Lauren's分型、肿瘤直径和CA125与胃癌淋巴结转移现象之间存在密切相关性。但是,只有BMI及肿瘤浸润深度是胃癌淋巴结转移现象的独立危险因素。本研究还发现,随着BMI数值的增加,淋巴结转移的阳性率也随之增加。BMI和浸润深度对淋巴结转移现象预测能力的特异度分别为76.84%和53.68%,敏感度均为63.16%。当将两者结合对淋巴结转移现象进行预测,发现其敏感度和特异度分别为63.16%和88.36%,曲线下面积为75.76%。并且,相比于BMI≥24 kg/m2的患者,BMI<24 kg/m2的患者3年总生存率明显较高。
近年来,许多研究表明,高B M I 不仅增加了如高血压、糖尿病和冠心病等慢性疾病的风险,还增加了恶性肿瘤的发病率[15-16]。这可能与瘦素(l e pt i n)、胰岛素样生长因子1(insul i n l i k e g r o w t h f a c t o r 1,I G F-1)以及脂联素(adiponectin)等有关。据报道,BMI高的人体内瘦素含量是普通人的4 倍,这种差异与肿瘤发病率之间也存在某种关联,特别是食管癌[17-18],研究[19]表明其对直肠癌的侵袭和转移也有直接影响;同时,IGF-1已被证实具有明显的促肿瘤作用,能促进肿瘤的增殖,增加结肠癌和前列腺癌的发病率[20-21];而研究[22-23]表明脂联素与食管癌的发病率呈负相关,与其具有一定的调节血管反应、炎症、细胞增殖和组织重塑的能力有关。但目前,所有这些具体机制仍在研究中。随着欧美肥胖的增加,肥胖对胃癌患者术后并发症和长期生存率的影响越来越受到关注[24]。也有一些研究[25]发现与本研究类似的现象,BMI与胃癌患者的生存率之间具有一定的独立相关性,患者BMI越高,预后越差。但其具体机制仍尚未明确。通常用BMI来描述肥胖程度,关于BMI与淋巴结转移现象之间的关系的报道很少。而关于肿瘤浸润深度与胃癌淋巴结转移现象之间的关系,许多研究已经表明,两者之间的影响是通过许多微分子发挥作用的,其中比较重要的有醛脱氢酶1(aldehyde dehydrogenase 1,ALDH1)[26]、趋化因子受体4(chemokine receptor 4CXCR4)[27]、E-钙粘蛋白(E-cadherin)[28]等。并且这些分子与胃癌的预后之间也存在这密切的相关性[29-30]。
目前,胃癌的主要治疗方法仍然是手术与化疗相结合。胃癌根治术的主要手术步骤是病灶的根治性切除和胃周淋巴结的清扫,目前主要依据日本胃癌协会于2014年公布的胃周淋巴结切除标准展开手术操作[31]。然而,在淋巴结清扫的程度上尚存在一定的争议,一些有学者[32-33]认为胃癌患者接受D2淋巴结清扫可以获得相比于D1清扫更好的疗效;而有些研究[34-35]认为,过度的淋巴结清扫会有适得其反的结果,对患者的预后产生一定的危害。而在胃癌患者根治性手术淋巴结清扫程度存在争论的主要原因是每例患者的具体情况并非完全相同,并且清扫前无明确的参考指标协助术者提前依据患者情况制定个体化淋巴结清扫程度[36]。本研究的结果可能能够为解决这一问题提供一定的帮助,通过结合患者术前BMI、超声胃镜等所测及的肿瘤浸润深度等独立危险因素,对胃癌患者淋巴结转移阳性率进行一定的预测,从而制定较为个体化的手术方式。
本研究仍有一些局限性。首先,研究对象中的大多数患者被就诊时已经诊断为进展期胃癌,因此本研究结果可能更适用于进展期胃癌患者的术前评估。其次,本研究证实,BMI对预测淋巴结转移阳性率有较好的作用,但特异度低,因此,BMI只能作为淋巴结转移现象的一个重要筛选指标。将BMI与浸润深度两个因素相结合来预测淋巴结转移的阳性率,可能得到更准确的预测结果。最后,本研究未能进一步研究BMI影响胃癌患者淋巴结转移阳性率的具体机制,仍需进一步研究其具体机制从而明确BMI影响胃癌患者预后的具体 机制。
综上所述,BMI与胃癌淋巴结转移阳性率及预后密切相关。BMI与浸润深度结合对胃癌淋巴结转移的阳性率具有一定的预测能力。当术前一般资料提示胃癌患者具有较高的BMI和(或)电子胃镜、超声胃镜等结果提示更深的浸润深度时,患者更容易发生淋巴结转移现象。此外,BMI越高,胃癌患者预后越差。如果术前明确淋巴结转移的现象,对胃癌患者的治疗和预后的预测将起到重要作用。
[1]McGuire S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015[J]. Adv Nutr, 2016, 7(2):418-419. doi: 10.3945/an.116.012211.
[2]Chen W, Zheng R, Baade PD, et al. Cancer statistics in China,2015[J]. CA Cancer J Clin, 2016, 66(2):115-132. doi: 10.3322/caac.21338.
[3]Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2)[J]. Lancet, 2015, 385(9972):977-1010. doi: 10.1016/S0140-6736(14)62038-9
[4]周潮平, 汤代彬, 汪大田, 等. 单中心186例早期胃癌区域淋巴结转移特征与预后分析[J]. 中国普通外科杂志, 2019, 28(10):1221-1227. doi:10.7659/j.issn.1005-6947.2019.10.009. Zhou CP, Tang DB, Wang DT, et al. Characteristics of lymph metastasis and prognostic analysis of 186 patients with early gastric cancer from a single center[J]. Chinese Journal of General Surgery, 2019, 28(10):1221-1227. doi:10.7659/j.issn.1005-6947.2019.10.009.
[5]Chu X, Yang ZF. Impact on survival of the number of lymph nodes resected in patients with lymph node-negative gastric cancer[J]. World J Surg Oncol, 2015, 13:192. doi: 10.1186/s12957-015-0602-x.
[6]Cao Y, Xiong L, Deng S, et al. The effect of perigastric lipolymphatic tissue grouping by surgeon on the number of pathologic sampled lymph nodes after radical gastrectomy[J]. Medicine (Baltimore), 2018, 97(27):e11411. doi: 10.1097/MD.0000000000011411.
[7]Serizawa A, Taniguchi K, Yamada T, et al. Successful conversion surgery for unresectable gastric cancer with giant para-aortic lymph node metastasis after downsizing chemotherapy with S-1 and oxaliplatin: a case report[J]. Surg Case Rep, 2018, 4(1):88. doi: 10.1186/s40792-018-0494-4.
[8]Kodera Y, Fujitani K, Fukushima N, et al. Surgical resection of hepatic metastasis from gastric cancer: a review and new recommendation in the Japanese gastric cancer treatment guidelines[J]. Gastric Cancer, 2014, 17(2):206-212. doi: 10.1007/s10120-013-0299-x.
[9]Jiao XG, Deng JY, Zhang RP, et al. Prognostic value of number of examined lymph nodes in patients with node-negative gastric cancer[J]. World J Gastroenterol, 2014, 20(13):3640. doi: 10.3748/wjg.v20.i13.3640.
[10]中国超重肥胖医学营养治疗专家共识编写委员会. 中国超重/肥胖医学营养治疗专家共识(2016年版)[J]. 中华糖尿病杂志, 2016, 8(9):525-540. doi:10.3760/cma.j.issn.1674-5809.2016.09.004. China Expert Consensus Committee on Medical Nutrition Therapy for Overweight and Obesity. China expert consensus on medical nutrition therapy for overweight and obesity (2016 edition)[J]. Chinese Journal of Diabetes Mellitus, 2016, 8(9):525-540. doi:10.3760/cma.j.issn.1674-5809.2016.09.004.
[11]Li LM, Feng LY, Chen XH, et al. Gastric heterotopic pancreas and stromal tumors smaller than 3 cm in diameter: clinical and computed tomography findings[J]. Cancer Imaging, 2018, 18(1):26. doi: 10.1186/s40644-018-0161-9.
[12]赵群, 李勇, 胡子龙, 等. 多层螺旋CT对胃癌术前分期及肿瘤最长径的评估价值[J]. 中华胃肠外科杂志, 2015, 18(3):227-231. doi:10.3760/cma.j.issn.1671-0274.2015.03.007. Zhao Q, Li Y, Hu ZL, et al. Value of the preoperative TNM staging and the longest tumor diameter measurement of gastric cancer evaluated by MSCT[J]. Chinese Journal of Gastrointestinal Surgery, 2015, 18(3):227-231. doi:10.3760/cma.j.issn.1671-0274.2015.03.007.
[13]杨朝美, 杨晏. PGR与血清肿瘤标记物联合检测在胃癌诊断的临床意义[J]. 中国普通外科杂志, 2018, 27(4):523-527. doi:10.3978/j.issn.1005-6947.2018.04.020. Yang CM, Yang Y. Clinical significance of combined detection of PGR and serum tumor markers in diagnosis of gastric cancer[J]. Chinese Journal of General Surgery, 2018, 27(4):523-527. doi:10.3978/j.issn.1005-6947.2018.04.020.
[14]Jin EH, Lee DH, Jung SA, et al. Clinicopathologic factors and molecular markers related to lymph node metastasis in early gastric cancer[J]. World J Gastroenterol, 2015, 21(2):571-577. doi: 10.3748/wjg.v21.i2.571.
[15]Misleh JG, Santoro P, Strasser JF, et al. Multidisciplinary management of gastric cancer[J]. Surg Oncol Clin N Am, 2013, 22(2):247-264. doi: 10.1016/j.soc.2012.12.013.
[16]Park J, Morley TS, Kim M, et al. Obesity and cancer--mechanisms underlying tumour progression and recurrence[J]. Nat Rev Endocrinol, 2014, 10(8):455-465. doi: 10.1038/nrendo.2014.94.
[17]Knight BB, Oprea-Ilies GM, Nagalingam A, et al. Survivin upregulation, dependent on leptin-EGFR-Notch1 axis, is essential for leptin-induced migration of breast carcinoma cells[J]. Endocr Relat Cancer, 2011, 18(4):413-428. doi: 10.1530/ERC-11-0075.
[18]Comstock SS, Hortos K, Kovan B, et al. Adipokines and obesity are associated with colorectal polyps in adult males: a cross-sectional study[J]. PLoS One, 2014, 9(1):e85939. doi: 10.1371/journal.pone.0085939.
[19]Wang D, Chen J, Chen H, et al. Leptin regulates proliferation and apoptosis of colorectal carcinoma through PI3K/Akt/mTOR signalling pathway[J]. J Biosci, 2012, 37(1):91-101. doi: 10.1007/s12038-011-9172-4.
[20]Uchiyama T, Takahashi H, Sugiyama M, et al. Leptinreceptor is involved in STAT3 activation in human colorectal adenoma[J]. Cancer Sci, 2011, 102(2):367-372. doi: 10.1111/j.1349-7006.2010.01803.x.
[21]Sikalidis AK, Varamini B. Roles of hormones and signaling molecules in describing the relationship between obesity and colon cancer[J]. Pathol Oncol Res, 2011, 17(4):785-790. doi: 10.1007/s12253-010-9352-9.
[22]Price AJ, Allen NE, Appleby PN, et al. Insulin like growth factor-I concentration and risk of prostate cancer: results from the European Prospective Investigation into Cancer and Nutrition[J]. Cancer Epidemiol Biomarkers Prey, 2012, 21(9):1531-1541. doi: 10.1158/1055-9965.EPI-12-0481-T.
[23]Kato M, Watabe K, Hamasaki T, et al. Association of low serum adiponectin levels with erosive esophagitis in men: an analysis of 2405 subjects undergoing physical check-ups[J]. J Gastroenterol, 2011, 46(12):1361-1367. doi: 10.1007/s00535-011-0453-3.
[24]Lee SS, Ryu SW, Kim IH, et al. Impact of gender and body mass index on surgical outcomes following gastrectomy: an Asia-Pacific perspective[J]. Chin Med J (Engl), 2012, 125(1):67-71.
[25]Lianos GD, Bali CD, Glantzounis GK, at al. BMI and lymph node ratio may predict clinical outcomes of gastric cancer[J]. Future Oncol, 2014, 10(2):249-255. doi: 10.2217/fon.13.188.
[26]Li XS, Xu Q, Fu XY, et al. ALDH1A1 overexpression is associated with the progression and prognosis in gastric cancer[J]. BMC Cancer, 2014, 14:705. doi: 10.1186/1471-2407-14-705.
[27]Sayasith K, Sirois J. Expression and regulation of stromal cellderived factor-1 (SDF1) and chemokine CXC motif receptor 4 (CXCR4) in equine and bovine preovulatory follicles[J]. Mol Cell Endocrinol, 2014, 391(1/2):10-21. doi: 10.1016/j.mce.2014.04.009.
[28]Otsuki T, Fujimoto D, Hirono Y, et al. Thrombin conducts epithelialmesenchymal transition via proteaseactivated receptor1 in human gastric cancer[J]. Int J Oncol, 2014, 45(6):2287-2294. doi: 10.3892/ijo.2014.2651.
[29]Piao S, Ojha R, Rebecca VW, et al. ALDH1A1 and HLTF modulate the activity of lysosomal autophagy inhibitors in cancer cells[J]. Autophagy, 2017, 13(12):2056-2071. doi: 10.1080/15548627.2017.1377377.
[30]赵艳, 金鑫, 李楠, 等. ALDH1、CXCR4、E-cadherin在胃癌组织中的表达及与淋巴结转移的关系[J]. 南方医科大学学报, 2016, 36(10):1390-1395. doi:10.3969/j.issn.1673-4254.2016.10.16. Zhao Y, Jin X, Li N, et al. Expression of ALDH1, CXCR4 and E- cadherin in castric carcinoma and their roles in lymphatic metastasis[J]. Journal of Southern Medical University, 2016, 36(10):1390-1395. doi:10.3969/j.issn.1673-4254.2016.10.16.
[31]Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4)[J]. Gastric Cancer, 2017, 20:1-19.
[32]Jo MJ, Park JY, Song JS, et al. Biopathologic features and clinical significance of micrometatasis in the lymph node of early gastric cancer[J]. World J Gastroenterol, 2015, 21(2):667-674. doi: 10.3748/wjg.v21.i2.667.
[33]Bravo Neto GP, dos Santos EG, Victer FC, et al. Lymph node metastasis in early gastric cancer[J]. Rev Col Bras Cir, 2014, 41(1):11-17. doi: 10.1590/s0100-69912014000100004.
[34]Chang JS, Koom WS, Lee Y, et al. Postoperative adjuvant chemoradiotherapy in D2-dissected gastric cancer: is radiotherapy necessary after D2-dissection?[J]. World J Gastroenterol, 2014, 20(36):12900-12907. doi: 10.3748/wjg.v20.i36.12900.
[35]Alshahrani AS, Yoo MW. Gastric cancer in pregnancy: is laparoscopic gastrectomy with lymph nodedissection feasible and safe?[J]. Ann Surg Treat Res, 2017, 92(1):51-53. doi: 10.4174/astr.2017.92.1.51.
[36]Sakcak I, Yıldız BD, Avşar FM, et al. Does N ratio affect survival in D1 and D2 lymph node dissection for gastric cancer?[J]. World J Gastroenterol, 2011, 17(35):4007-4012. doi: 10.3748/wjg.v17.i35.4007.
