结直肠癌为全球第三大最常见的恶性肿瘤,据报道仅2018年全球新增结直肠癌病例180万,死亡86万例[1]。近10年来,由于生活和饮食习惯的变化,结直肠癌的发病率迅速上升[2-3]。癌细胞转移是目前结直肠癌致死的主要原因,大约60%的结直肠癌患者后期会发生转移[4]。尽管结直肠癌的标准治疗方法可以使早期结直肠癌患者的病情有所改善,但对中晚期结直肠癌患者疗效并不明显,5年生存率仅为13.9%[5-6]。因此,有必要了解中晚期结直肠癌进展与转移的机制,希望能找到一种新的可以降低晚期结直肠患者耐药的治疗靶点。越来越多的证据表明转录因子在对肿瘤的进展中发挥重要作用,比如FOXP家族(FOXP1、FOXP2、FOXP3和FOXP4)作为其中一类转录因子,研究发现其在多种肿瘤的生长、发生和转移中发挥重要作用[7-9]。已证实FOXP1低表达的结直肠癌,其肿瘤细胞增殖能力强,生存期较差[10]。而FOXP4在结直肠癌中的调节机制目前尚未报道,因此本研究通过收集结直肠癌患者的临床标本,分析FOXP4在癌组织和癌旁组织中的表达水平,并通过细胞与分子生物学实验进一步分析相关的分子机制。
1 材料与方法
1.1 实验材料
HT-29细胞、CaCo2细胞、HT-29和CaCo2耐药细胞均购自盖宁生物;DMEM 细胞培养基、胎牛血清购自Gibco公司;XAV-939以及CCK8试剂盒购自Selleck公司;psPAX2、pMD2G、plKO.1-NO、pLKO.1-siFOXP4、pLenti-3F以及pLenti-F O X P 4 购自生物风有限公司;F O X P 4 引物、GAPDH引物购自武汉擎科生物有限公司;RNA提取试剂盒购自Takara公司;RNA逆转录试剂盒(Revert Aid First Strand cDNA Synthesis Kit)以及Universal SYBR® qPCR Master Mix试剂盒购自南京诺唯赞生物科技有限公司;一抗GAPDH、β-catenin及辣根过氧化物酶标记的羊抗兔/鼠二抗购自三鹰生物科技有限公司;Western blot试剂盒及BCA蛋白浓度测量试剂盒购自碧云天生物技术公司;免疫组化试剂盒以及双荧光素酶试剂盒购自Sigma公司。
1.2 实验方法
1.2.1 细胞培养 239T 细胞、LOVO 细胞、CaCo2细胞、DLD1 细胞、SW48 细胞、HCT116 细胞均培养于含10%FBS-DMEM 中,5% CO2,37 ℃。
1.2.2 组织来源 于西安交通大学医学部附属三二〇一医院收集50 例确诊为结直肠癌的患者临床信息,取其癌组织和癌旁组织。取出样本后立即置于液氮中冷冻,然后置于-80 ℃保存。
1.2.3 病毒包装 收集对数期生长293T 细胞,接种于6 cm 培养皿中,待细胞融合度达到70%时进行转染。 首先将质粒以pLent-3F/pLent-FOXP4/plKO.1-NO/pLKO.1-siFOXP4:psPAX2: pMD2G=4:3:1 的比例混合,转染至293T 细胞,6 h后更换新鲜DMEM 培养基,48 h 收细胞上清。
1.2.4 病毒感染及稳定敲减细胞系筛选 收集处于对数生长期的结直肠癌细胞系,待细胞融合度达到80% 时进行转染,严格按照Lipofectamine 3000 说明书进行慢病毒感染,24 h 后更换至新鲜培养基。并进一步采用嘌呤霉素(2 mg/L)持续筛选,2 周后,采用Western blot,RT-PCR 检测上述细胞的过表达以及敲减的效率。
1.2.5 RT-PCR 检测 首先使用TRNA 提取试剂盒提取RNA;进一步将RNA 逆转录生成cDNA。以GAPDH 为内参,GAPDH 正向引物为5'-GGC ATG GAC TGT GGT CAT GAG-3',反向引物为5'-TGC ACC ACC AAC TGT TAG C-3';FOXP4 正向引物为5'-CGA CAT GAT GGT GGA ATC TG-3',反向引物为5'-TGT TTG CTG TCA TTG TTC CC-3',所有基因的相对表达水平用2-∆CT 法计算。
1.2.6 Western blot 检测 加入RIPA 裂解液处理细胞,待细胞充分裂解后,4 ℃,离心10 min。取上清,BCA 法测定蛋白浓度,取等量的蛋白上样,运行SDS-PAGE 电泳。然后采用PDVF 膜转膜,5% 脱脂奶粉封闭,孵育FOXP4、β-catenin、GAPDH 一抗,室温孵育二抗,曝光显影。
1.2.7 CCK8 检测细胞活力 将处理好的LOVO细胞,SW48 细胞,SW480 细胞,HCT116 细胞接种于96 孔板中,接种密度为2.0×103 个。分别于0、12、24、48、72 h 避光加入CCK-8 溶液30 μL;孵育3 h 后,采用多功能酶标仪检测450 nm 波长处的吸光度值。
1.2.8 Transwell 检测细胞的迁移能力 将处理好 的LOVO 细 胞,SW48 细 胞,SW480 细 胞,HCT116 细胞培养于Transwell 小室的上层,同时下层添加500 μL 含有10%FBS 的DMEM 培养基。培养48 h 后,用棉签去除上室的细胞,室温下用0.1% 结晶紫染色30 min。最后,对迁移的细胞进行计数,并用光学显微镜拍照。每个独立的实验至少重复3 次。
1.2.9 免疫组化检测 组织切片入放入65 ℃烤箱中烤1.5 h,切片在二甲苯放置10 min,更换二甲苯放置10 min,将切片依次放入100% 乙醇、95%乙醇、80% 乙醇中、纯化水各5 min,将切片放入修复盒中加入抗原修复液,高压锅加热到自动放气,2 min 后离开热源,自然冷却。弃去抗原修复液,将切片用PBS 淋洗,0.5%Triton X-100 室温通透20 min,将切片移入湿盒中加入新鲜配制的3% 双氧水,室温孵育10 min,PBS 充分淋洗,浸洗3 次,每次3 min,吸水纸吸干PBS,在玻片上滴加山羊血清,室温封闭30 min,每张玻片滴加足够量的稀释好的一抗(FOXP4 1:300),放入湿盒,4 ℃孵育过夜,滴加兔二抗工作液,室温孵育1 h,PBS 充分淋洗,DAB 显色10 min,PBS 冲洗2 min,苏木精复染3 min,盐酸酒精分化,反蓝,PBS 冲洗1 min 脱水、透明、封片、光学显微镜下拍照。
1.2.10 ChIP 实验检测 取出经过实验要求处理的细胞,加入1% 多聚甲醛,37 ℃孵育10 min,加甘氨酸,混匀后,在室温下放置5 min。吸尽培养基,用冰冷的PBS 清洗细胞2 次。细胞刮刀收集细胞于15 mL 离心管中,2 000 r/min,离心 5 min,收集细胞,加入500 μL SDS 裂解缓冲液,超声破碎,1 200 r/min,4 ℃,离心10 min。取上清,其中1 管中加入1 μL FOXP4 抗体,另1 管中加对照IgG,4 ℃颠转过夜。孵育过夜后,每管中加入 60 μL ProteinA/G,4 ℃, 孵 育2 h。700 r/min离心1 min,除去上清,每管中加入20 μL NaCl溶液,混匀,65 ℃解交联过夜,每管加入1 μL RNaseA,37 ℃孵育1 h,每管加入10 μL EDTA,20 μL Tris-HCl(pH6.5),2 μL 10 mg/mL 蛋白酶K,回收DNA 片段。
1.2.11 荧光素酶检测 将野生型以及突变型的β-catenin 的启动子质粒转染到相应的细胞系中,48 h 后,使用双荧光素酶报告系统检测荧光素酶活性。
1.3 统计学处理
每组实验重复至少3次,应用SPSS 14.0进行统计分析,计量数据用均数±标准差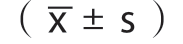 表示,组间比较均采用t检验,P<0.05为差异有统计学意义。
表示,组间比较均采用t检验,P<0.05为差异有统计学意义。
2 结 果
2.1 FOXP4 在结直肠癌组织中的表达及作用分析
采用免疫组化和RT-PCR检测癌组织和癌旁组织中FOXP4的相对表达量(图1A-B),结果显示结直肠癌患者癌组织中FOXP4的表达量明显高于癌旁组织(P<0.01)。采用GEIPA(http://gepia.cancer-pku.cn)数据库分析FOXP4在结直肠癌中的表达量以及对结直肠癌患者的生存期的影响(图1 C-D),结果显示,癌组织中的F O X P 4 表达量明显高于正常组织(P <0.0 5),且高表达F O X P 4 的患者其总体生存率明显较差(P<0.05)。据上述结果推测FOXP4在结直肠癌进展中可能发挥一定作用。
比较结直肠癌组中F O X P 4 表达与不同病理特征发现,FOXP4的表达在不同性别、年龄段的患者中无明显差异(均P>0.05),在不同肿瘤大小、分化程度、T分期、N分期及M分期的患者中差异具有统计学意义(均P<0.05),其在肿瘤大小>1 cm、分化程度中低、T3~4期、及高N和M1期的患者中表达明显增高(表1)。
2.2 FOXP4 表达水平对结直肠癌细胞的增殖的影响
进一步探究FOXP4对结直肠癌进展的影响,首先使用R T-P C R 检测结肠癌细胞系L O V O,SW48,SW480,HCT116,CaCo2以及DLD1中FOXP4的表达量(图2A),结果显示,LOVO,S W 4 8 细胞F O X P 4 表达量相对降低,H C T 1 1 6、S W 4 8 0 细胞F O X P 4 表达量相对较高。基于该结果,构建SW48-空载体、SW48-FOXP4、LOVO-空载体、LOVO-FOXP4细胞系以及SW480-阴性对照序列、SW480-siFOXP4、HCT116-阴性对照序列、HCT116-siFOXP4细胞系;采用RT-PCR证实了FOXP4在结直肠癌细胞系中的过表达及敲减效率(图2B-C)。进一步使用CCK8检测FOXP4对结直肠癌细胞系增殖能力的影响(图2D-G),结果表明,FOXP4过表达可明显增加结直肠癌细胞的增殖能力(均P<0.01);FOXP4低表达可明显降低结直肠癌细胞的增殖能力(均P<0.01)。
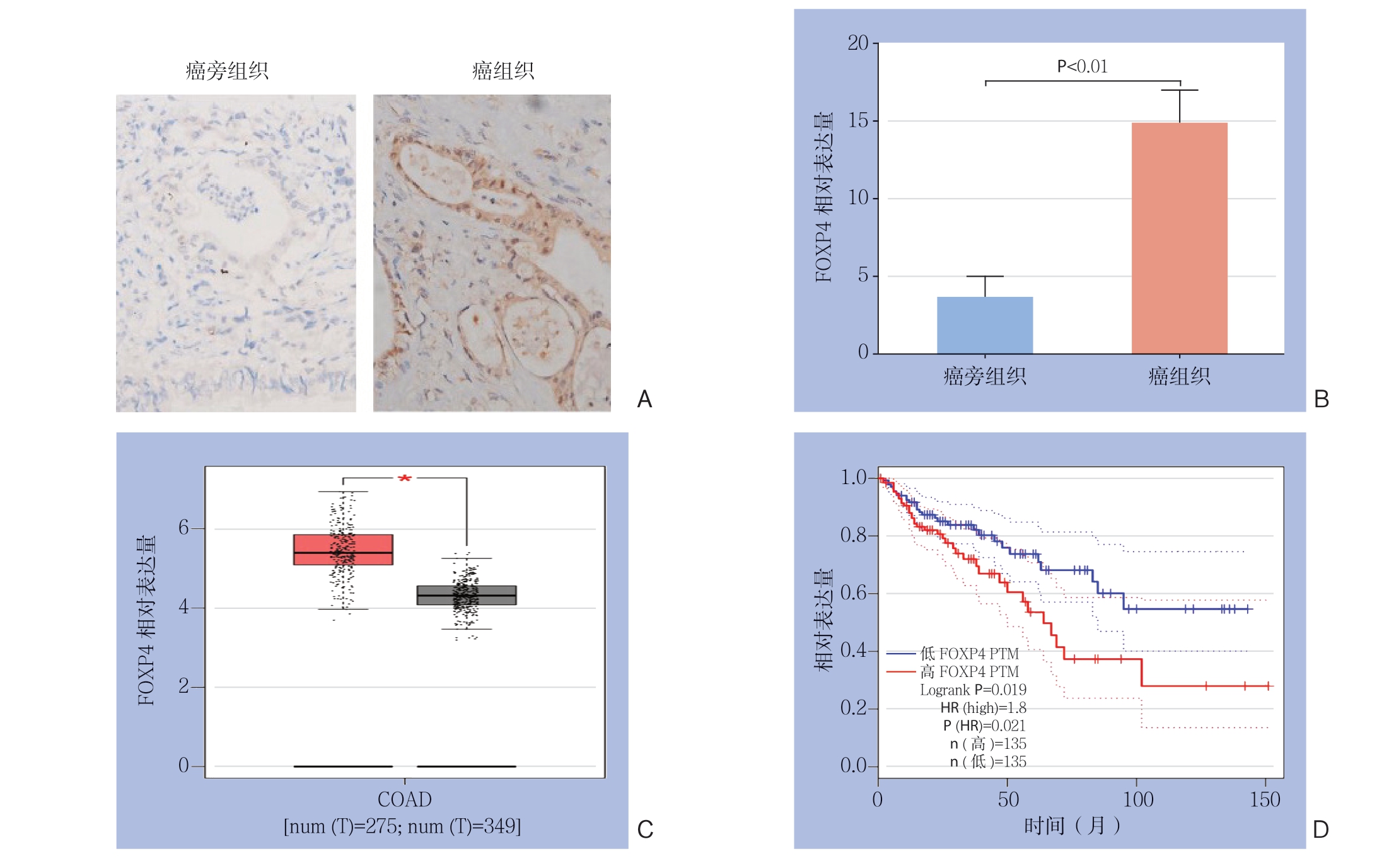
图1 FOXP4 在结直肠癌中表达的检测与分析 A:免疫组化检测结直肠癌组织与癌旁组织中FOXP4 的表达(×200);B:RT-PCR 检测结直肠癌组织和癌旁组织中FOXP4 的表达;C:GEPIA 数据库分析结直肠癌患者FOXP4 的表达; D:GEPIA 数据库分析结直肠癌患者FOXP4 的表达量与总体生存率的关系
Figure 1 Determination and analysis of FOXP4 expression in colorectal cancer A: Immunohistochemical attaining for FOXP4 expressions in colorectal cancer tissues and adjacent tissues (×200); B: RT-PCR detection of FOXP4 expressions in colorectal cancer tissues and adjacent tissues; C: GEPIA database analysis of the expression level of FOXP4 in patients with rectal cancer; D: GEPIA database analysis of the relationship between the expression level of FOXP4 and overall survival rate in patients with colorectal cancer
表1 FOXP4 表达与结直肠癌患者临床病理特征的关系[n(%)]
Table 1 Relations of FOXP4 expression with the clinicopathologic characteristics of colorectal cancer patients [n (%)]
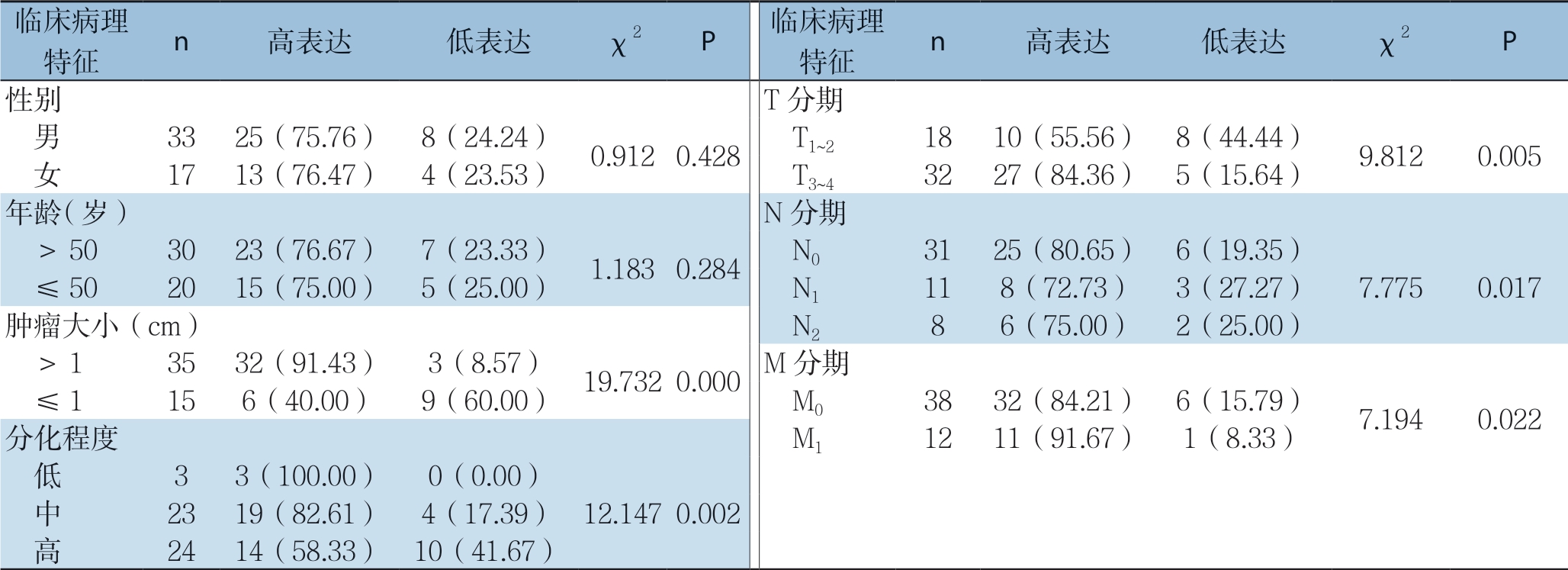
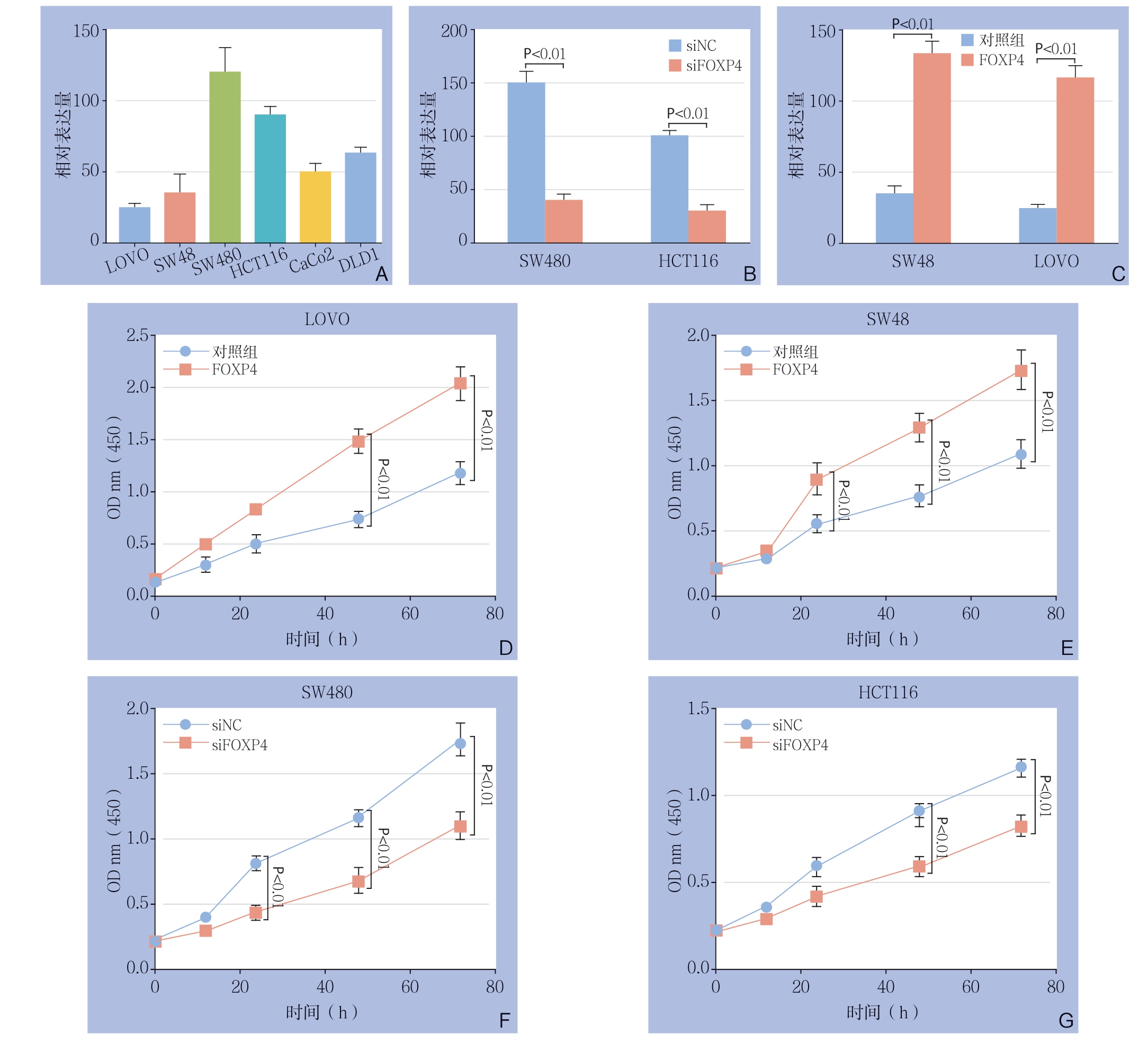
图2 FOXP4 表达量对结直肠癌细胞增殖能力的影响 A:RT-PCR 检测结直肠癌细胞系中FOXP4 的表达量;B:RT-PCR检测结直肠癌细胞系中FOXP4 的过表达效率;C:RT-PCR 检测结直肠癌细胞系中FOXP4 的敲减效率;D:CCK8 检测FOXP4 过表达对LOVO 细胞增殖能力的影响;E:CCK8 检测FOXP4 过表达对SW48 细胞增殖能力的影响;F:CCK8 检测FOXP4 敲减对SW480 细胞增殖能力的影响;G:CCK8 检测FOXP4 敲减对HCT116 细胞增殖能力的影响
Figure 2 The influence of FOXP4 expression level on the proliferative ability of colorectal cancer cells A: RT-PCR detection of FOXP4 expression levels in different colorectal cancer cell lines; B: RT-PCR detection of FOXP4 overexpression efficiency in colorectal cancer cell lines; C: RT-PCR detection of FOXP4 knockdown efficiency in colorectal cancer cell lines; D: CCK8 detection of the effect of FOXP4 overexpression on the proliferation of LOVO cells; E: CCK8 detection of the effect of FOXP4 overexpression on the proliferation of SW48 cells; F: CCK8 detection of the effect of FOXP4 knockdown on the proliferation of SW480 cells; G: CCK8 detection of the effect of FOXP4 knockdown on the proliferation of SW480 cells
2.3 FOXP4 促进结直肠癌细胞的迁移能力
进一步探讨FOXP4对结直癌细胞系迁移能力的影响,结果显示,过表达FOXP4后,LOVO细胞和SW48细胞的迁移能力明显增加(均P<0.01)(图3A-D);FOXP4敲减后,SW480和HCT116细胞的迁移能力明显下降(均P<0.01)(图3E-H)。
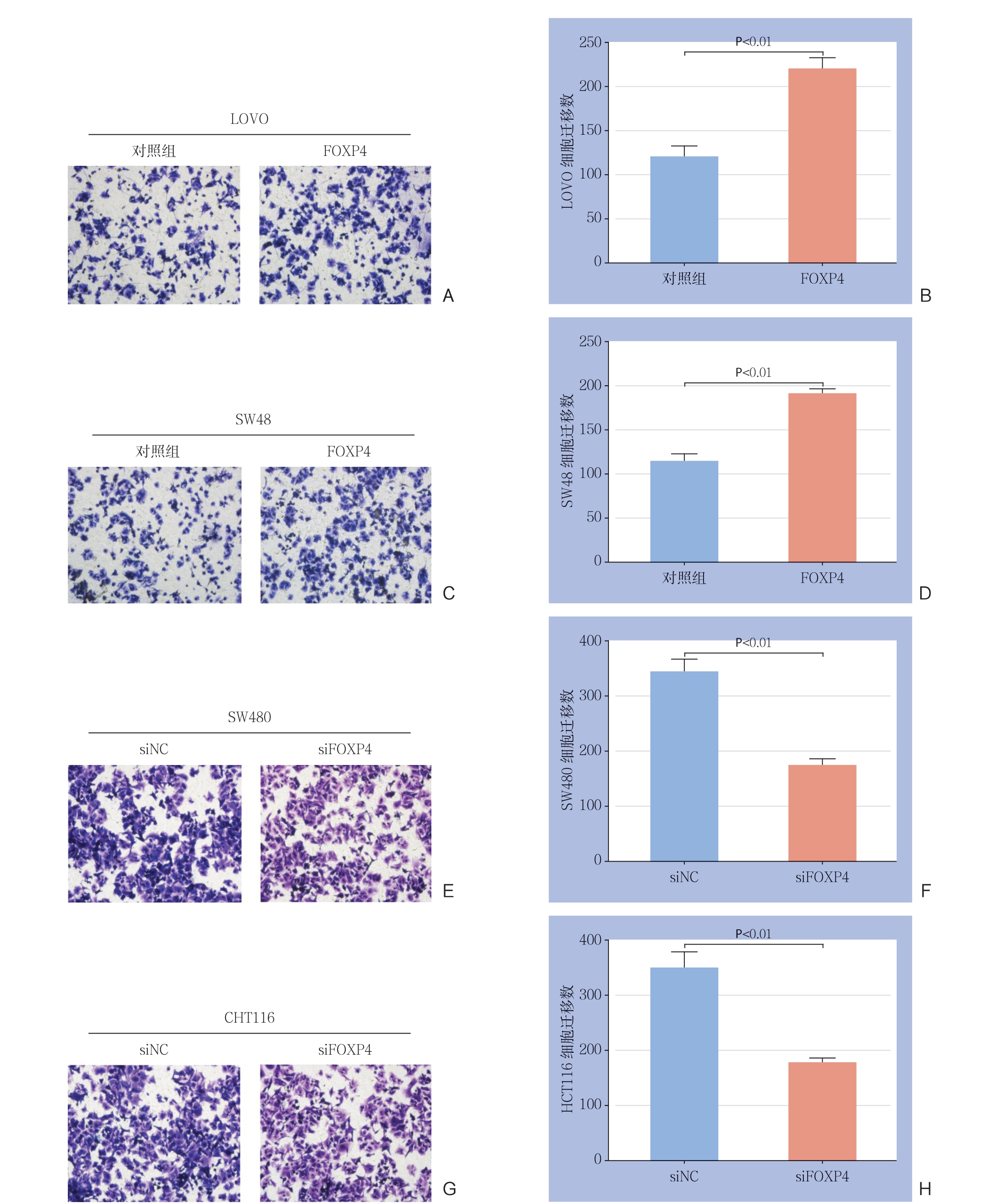
图3 FOXP4 表达量对结直肠癌细胞迁移能力的影响 A-B:Tranwell 检测过表达FOXP4 后,LOVO 细胞的迁移能力; C-D:Tranwell 检测过表达FOXP4 后,SW48 细胞的迁移能力;E-F:Tranwell 检测FOXP4 敲减后,SW480 细胞的迁移能力;G-H:Tranwell 检测FOXP4 敲减后,HCT116 细胞的迁移能力
Figure 3 The effect of FOXP4 expression level on the migration ability of colorectal cancer cells A-B: Tranwell assay for the migration ability of LOVO cells after FOXP4 overexpression; C-D: Tranwell assay for the migration ability of SW48 cells after FOXP4 overexpression; E-F: Tranwell assay for the migration ability of SW480 cells after FOXP4 knockdown; G-H: Tranwell assay for the migration ability of HCT116 cells after FOXP4 knockdown
2.4 FOXP4 对β-catenin 转录水平的影响
上述实验初步证明了FOXP4可调节结直肠癌细胞的增殖、转移,接下来,进一步探究其涉及到的分子机制。首先,采用AnimalTFDB 3.0数据库预测F O X P 4 的靶启动子(图4 A),结果发现FOXP4有可能与β-catenin启动子结合。进一步采用ChIP实验证明了FOXP4可识别β-catenin启动子区域(图4B)。此外,RT-PCR和Western blot分别从转录和翻译水平验证了FOXP4过表达可明显增加β-catenin的表达(图4C-E)。最后,构建野生型和突变型的β-catenin荧光素酶报告基因(图4D),结果显示,FOXP4只能识别野生型的β-catenin的序列(图4G-H)。综合以上结果,提示FOXP4可识别调控β-catenin的转录水平。
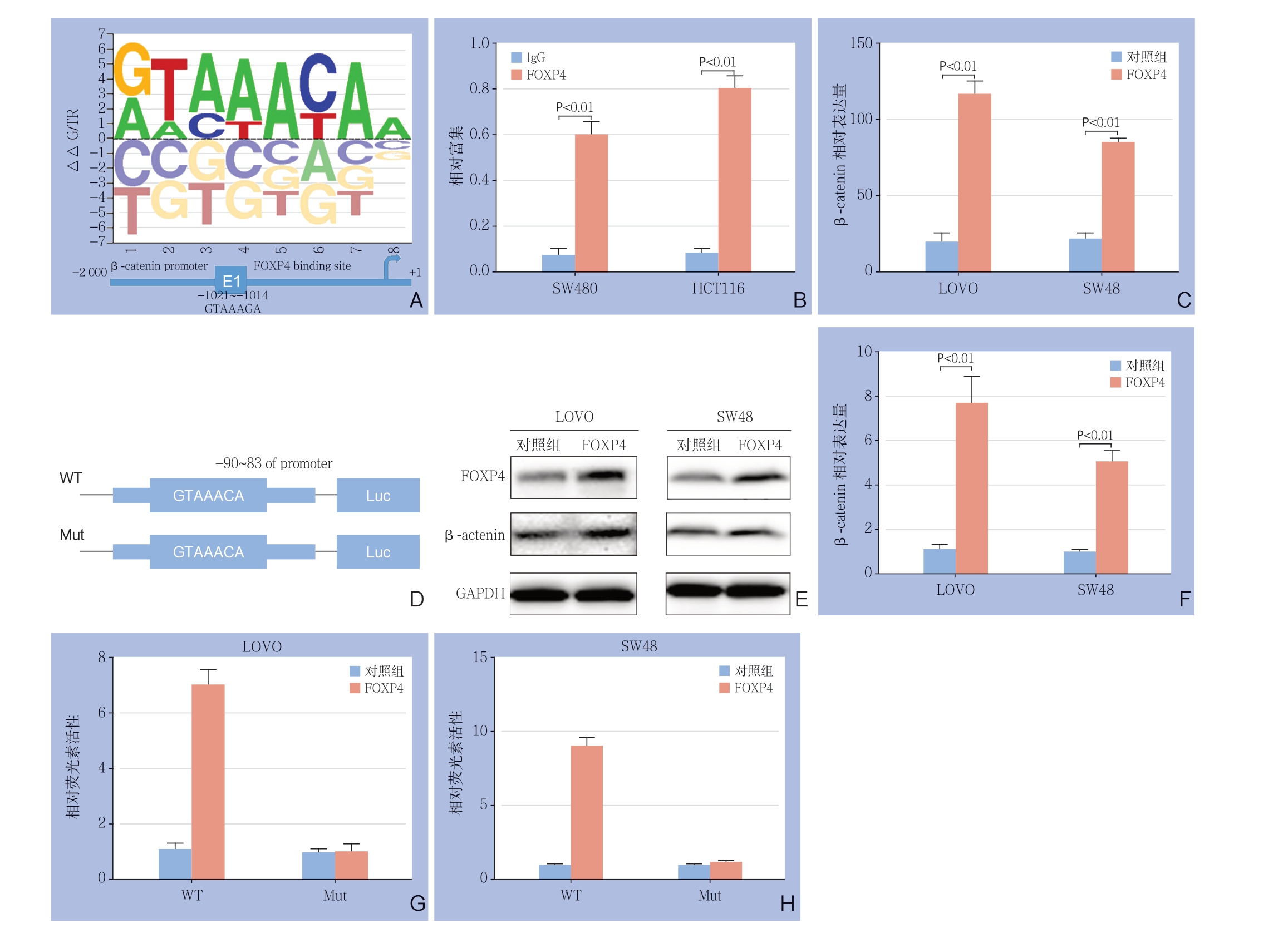
图4 FOXP4 对β-catenin 转录水平的影响 A:AnimalTFDB 3.0 预测FOXP4 结合β-catenin 启动子序列;B:ChIP 验证FOXP4 与β-catenin 的相互作用;C:RT-PCR 检测FOXP4 过表达对β-catenin 的mRNA 水平的影响;D:野生型以及突变型的β-catenin 启动子荧光素酶报告质粒的构建;E-F:Western blot 检测FOXP4 过表达后对β-catenin 的蛋白水平的影响;G-H:荧光素酶报告实验检测FOXP4 对野生型以及突变型的β-catenin 转录活性的影响
Figure 4 Effect of FOXP4 on β-catenin transcription level A: AnimalTFDB3.0 predicting the FOXP4 binding to the promoter sequence of β-catenin; B: ChIP verification of the interaction between FOXP4 and β-catenin; C: RT-PCR detection of the effect of FOXP4 overexpression on the mRNA expression level of β-catenin; D: Construction of luciferase reporter plasmids of wild and mutant type of β-catenin promoter; E-F: Western blot analysis of the effect of FOXP4 overexpression on protein expression level of β-catenin; G-H: Luciferase report assay for influence of FOXP4 on transcriptional activities of the wild and mutant type of β-catenin
2.5 结直肠癌细胞中β-catenin 与FOXP4 的关系
为进一步确定β-c a t e n i n 是否参与介导了FOXP4所引起的结直肠癌细胞增殖迁移能力的变化,使用β-catenin抑制剂XAV-939处理过表达F O X P 4 的结直肠癌细胞,结果显示,F O X P 4 过表达对结直肠癌细胞增殖与迁移能力的增强作用均被取消(均P<0.05)(图5)。上述实验结果说明β-catenin介导了FOXP4对结直肠癌细胞的调控 作用。
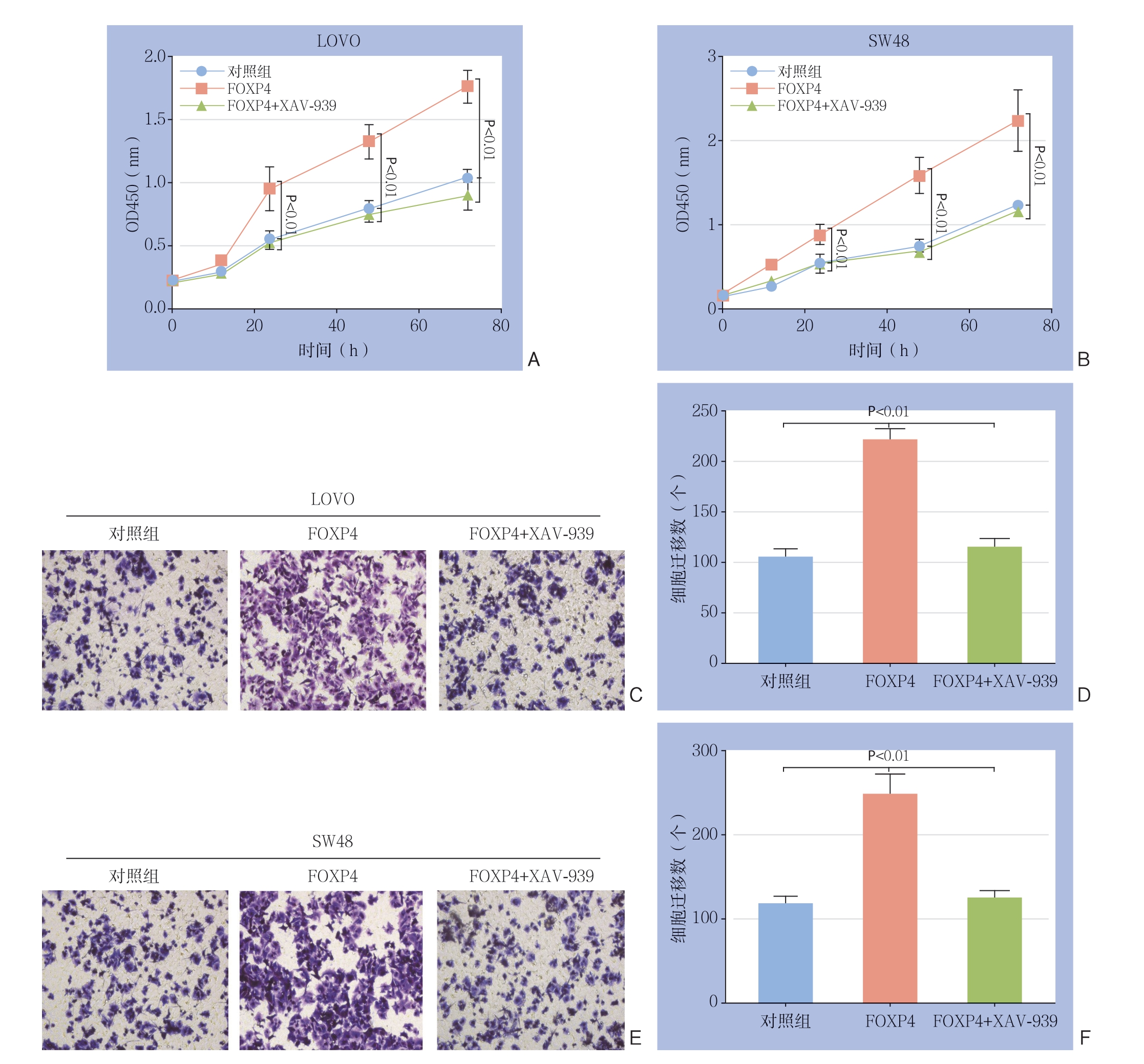
图5 结直肠癌细胞中β-catenin 与FOXP4 的关系 A:CCK8 检测β-catenin 抑制剂对SW48 细胞增殖能力的影响; B:CCK8 检测β-catenin 抑制剂对LOVO 细胞增殖能力的影响;C-D:Transwell 检测β-catenin 抑制剂对SW48 细胞迁移能力的影响;E-F:Transwell 检测β-catenin 抑制剂对LOVO 细胞迁移能力的影响
Figure 5 Relationship between β-catenin and FOXP4 in colorectal cancer cells A: CCK8 assay for the effect of β-catenin inhibitor on the proliferation of SW48 cells; B: CCK8 assay for the proliferation of LOVO cells; C-D: Transwell assay for the effect of β-catenin inhibitor on migration ability of SW48 cells; E-F: Transwell assay for the effect of β-catenin inhibitor on migration ability of LOVO cells
3 讨 论
中晚期结直肠癌的恶性增殖以及远处转移是导致结直肠癌患者死亡的主要原因,约有15%~25% 的结直肠癌患者出现肝转移[11-12]。目前肝转移灶的切除只可以提高部分患者(25%~40%)的5年生存率[13-14],所以目前急需探索可影响结直癌增殖、远处转移的因素。
FOXP转录因子家族可通过调节肿瘤细胞的上皮间充质转化影响肿瘤的细胞生物学表型。比如FOXP1可调控趋化因子CXCL的表达来控制乳腺癌细胞对淋巴结的侵润[15-16];同样在三阴性乳腺癌中,FOXP2可通过靶向GRP78增加肿瘤细胞的增殖与迁移能力[17];FOXP3特异性表达于T调节细胞中,参与调节肿瘤细胞的免疫耐受能力,最近也有文章报道FOXP3可通过抑制COX2的转录抑制结直肠癌干细胞的自我更新[18-21];FOXP4可通过调节转录因子Snail的活性来增强上皮间质转化(EMT)相关分子的表达,促进乳腺癌细胞的转移[22],而在肝细胞癌中的FOXP4可激活Slug的转录活性,促进肿瘤细胞迁移能力[23]。本研究发现,与癌旁正常组织相比,FOXP4在结直肠癌组织中表达显著上调,FOXP4高表达与患者不同临床特征具有显著性差异,同时高表达FOXP4的患者总体生存率可能降低。细胞层面发现FOXP4过表达可促进结直肠癌细胞的增殖和迁移,而敲低FOXP4可抑制结直肠癌细胞的增殖及迁移,这些实验结果表明FOXP4可能在结直肠肿瘤的进展中起关键作用。
在80%的结直肠癌患者中β-catenin处于过度激活状态[24-25],β-catenin是Wnt通路的靶向分子,其激活被认为是肿瘤形成过程的核心。另外还发现β-catenin/TCF信号在90%以上的结直肠癌中呈现高表达,而这与结直肠癌的不良预后息息相关[26-30]。然而目前关于转录因子FOXP4是如何影响结直肠癌进展的机制仍不清楚。本研究发现FOXP4可以识别β-catenin启动子区域,提示FOXP4可能通过调控β-catenin发挥促结直肠癌增殖和迁移的作用。进一步验证试验发现,过表达FOXP4可增加β-catenin基因及蛋白的表达,其仅可识别野生型β-catenin的序列。使用β-catenin抑制剂抑制其活性后发现,F O X P 4 过表达所促进的结直肠癌细胞增殖和迁移能力下降,表明FOXP4可能通过调控β-catenin活性促进结直肠癌细胞增殖和迁移。
尽管本研究首次揭示FOXP4在结直肠癌中的可能作用,但本实验结果仍需在动物层面进一步验证,同时FOXP4是如何调控β-catenin活性,其具体调控机制尚不清晰,这将是今后进一步研究的方向。
总之,本文证明了FOXP4可促进结直肠癌细胞的增殖、转移,在分子机制上,明确了FOXP4可能通过促进β-catenin的转录活性,增加结直肠癌细胞的增殖与转移能力,这为未来结直肠癌患者的治疗提供新的思路。
[1]Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020[J]. CA Cancer J Clin, 2020, doi: 10.3322/caac.21601. [Epub ahead of print]
[2]Riley JM, Cross AW, Paulos CM, et al. The clinical implications of immunogenomics in colorectal cancer: a path for precision medicine[J]. Cancer, 2018, 124(8):1650-1659. doi:10.1002/cncr.31214.
[3]Van Blarigan EL, Meyerhardt JA. Role of physical activity and diet after colorectal cancer diagnosis[J]. J Clin Oncol, 2015, 33(16):1825-1834. doi: 10.1200/JCO.2014.59.7799.
[4]Fakih MG. Metastatic colorectal cancer: current state and future directions[J]. J Clin Oncol, 2015, 33(16):1809-1824. doi: 10.1200/JCO.2014.59.7633.
[5]Ciombor KK, Wu C, Goldberg RM. Recent therapeutic advances in the treatment of colorectal cancer[J]. Annu Rev Med, 2015, 66:83-95. doi: 10.1146/annurev-med-051513-102539.
[6]Bardou M, Barkun A, Martel M. Effect of statin therapy on colorectal cancer[J]. Gut, 2010, 59(11):1572-1585. doi:10.1136/gut.2009.190900.
[7]Myatt SS, Lam WF. The emerging roles of forkhead box (Fox) proteins in cancer[J]. Nat Rev Cancer, 2007, 7(11):847-859. doi:10.1038/nrc2223.
[8]Nilsson J, Helou K, Kovács A, et al. Nuclear Janus-activated kinase 2/nuclear factor 1-C2 suppresses tumorigenesis and epithelial-tomesenchymal transition by repressing Forkhead box F1[J]. Cancer Res, 2010, 70(5):2020-2029. doi:10.1158/0008-5472.CAN-09-1677.
[9]Wei HJ, Nickoloff JA, Chen WH, et al. FOXF1 mediates mesenchymal stem cell fusion-induced reprogramming of lung cancer cells[J]. Oncotarget, 2014, 5(19):9514-9529. doi:10.18632/oncotarget.2413.
[10]De Smedt L, Palmans S, Govaere O, et al. Expression of FOXP1 and Colorectal Cancer Prognosis[J]. Lab Med, 2015, 46(4):299-311. doi:10.1309/LM7IHV2NJI1PHMXC.
[11]Carpizo DR, D'Angelica M. Liver resection for metastatic colorectal cancer in the presence of extrahepatic disease[J]. Ann Surg Oncol, 2009, 16(9):2411-2421. doi: 10.1245/s10434-009-0493-6.
[12]Rex DK, Kahi CJ, Levin B, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer[J]. Gastroenterology, 2006, 130(6):1865-1871. doi:10.1053/j.gastro.2006.03.013.
[13]Fong Y. Surgical therapy of hepatic colorectal metastasis[J]. CA Cancer J Clin, 1999, 49(4):231-255. doi: 10.3322/canjclin.49.4.231.
[14]Bentrem DJ, Dematteo RP, Blumgart LH. Surgical therapy for metastatic disease to the liver[J]. Annu Rev Med, 2005, 56:139-156. doi: 10.1146/annurev.med.56.082103.104630.
[15]Yu BH, Li BZ, Zhou XY, et al. Cytoplasmic FOXP1 expression is correlated with ER and calpain II expression and predicts a poor outcome in breast cancer[J]. Diagn Pathol, 2018, 13(1):36. doi: 10.1186/s13000-018-0715-y.
[16]De Silva P, Garaud S, Solinas C, et al. FOXP1 negatively regulates tumor infiltrating lymphocyte migration in human breast cancer[J]. EBioMedicine, 2019, 39:226-238. doi: 10.1016/j.ebiom.2018.11.066.
[17]Wu J, Liu P, Tang H, et al. FOXP2 Promotes Tumor Proliferation and Metastasis by Targeting GRP78 in Triple-negative Breast Cancer[J]. Curr Cancer Drug Targets, 2018, 18(4):382-389. doi: 10.2174/1568009618666180131115356.
[18]Masiuk KE, Laborada J, Roncarolo MG, et al. Lentiviral Gene Therapy in HSCs Restores Lineage-Specific Foxp3 Expression and Suppresses Autoimmunity in a Mouse Model of IPEX Syndrome[J]. Cell Stem Cell, 2019, 24(2):309-317. doi:10.1016/j.stem.2018.12.003.
[19]Stathopoulou C, Gangaplara A, Mallett G, et al. PD-1 Inhibitory Receptor Downregulates Asparaginyl Endopeptidase and Maintains Foxp3 Transcription Factor Stability in Induced Regulatory T Cells[J]. Immunity, 2018, 49(2):247-263. doi:10.1016/j.immuni.2018.05.006.
[20]Van Gool F, Nguyen MLT, Mumbach MR, et al. A Mutation in the Transcription Factor Foxp3 Drives T Helper 2 Effector Function in Regulatory T Cells[J]. Immunity, 2019, 50(2):362-377. doi:10.1016/j.immuni.2018.12.016.
[21]Liu S, Zhang C, Zhang K, et al. FOXP3 inhibits cancer stem cell self-renewal via transcriptional repression of COX2 in colorectal cancer cells[J]. Oncotarget, 2017, 8(27):44694-44704. doi:10.18632/oncotarget.17974.
[22]Ma T, Zhang J. Upregulation of FOXP4 in breast cancer promotes migration and invasion through facilitating EMT[J]. Cancer Manag Res, 2019, 11:2783-2793. doi: 10.2147/CMAR.S191641.
[23]Zhang G, Zhang G. Upregulation of FoxP4 in HCC promotes migration and invasion through regulation of EMT[J]. Oncol Lett, 2019, 17(4):3944-3951. doi:10.3892/ol.2019.10049.
[24]White BD, Chien AJ, Dawson DW. Dysregulation of Wnt/β-catenin signaling in gastrointestinal cancers[J]. Gastroenterology, 2012, 142(2):219-232. doi: 10.1053/j.gastro.2011.12.001.
[25]Walther A, Johnstone E, Swanton C, et al. Genetic prognostic and predictive markers in colorectal cancer[J]. Nat Rev Cancer, 2009, 9(7):489-499. doi: 10.1038/nrc2645.
[26]Peters U, Jiao S, Schumacher FR, et al. Identification of Genetic Susceptibility Loci for Colorectal Tumors in a Genome-Wide Metaanalysis[J]. Gastroenterology, 2013, 144(4):799-807. doi:10.1053/j.gastro.2012.12.020.
[27]Moon RT, Bowerman B, Boutros M, et al. The promise and perils of Wnt signaling through beta-catenin[J]. Science, 2002, 296(5573):1644-1646. doi:10.1126/science.1071549.
[28]Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors[J]. Cancer Treat Rev, 2018, 62:50-60. doi: 10.1016/j.ctrv.2017.11.002.
[29]Yu W, Ma Y, Shankar S, et al. SATB2/β-catenin/TCF-LEF pathway induces cellular transformation by generating cancer stem cells in colorectal cancer[J]. Sci Rep, 2017, 7(1):10939. doi: 10.1038/s41598-017-05458-y.
[30]Wang Z, Zhou L, Xiong Y, et al. Salinomycin exerts anti-colorectal cancer activity by targeting the β-catenin/T-cell factor complex[J]. Br J Pharmacol, 2019, 176(17):3390-3406. doi: 10.1111/bph.14770.
