甲状腺癌是最常见的内分泌系统恶性肿瘤,近年来,甲状腺癌发病率屡日剧增。甲状腺乳头状癌(papillary thyroid carcinoma,PTC)是甲状腺癌的常见病理类型,其分化程度较高,恶性程度低,总体预后良好,但侵袭型乳头状甲状腺癌有转移倾向,淋巴结转移较早,约占甲状腺恶性肿瘤的85%[1]。部分基因突变与PTC侵袭性相关,但非编码RNA在PTC发生和转移中的作用机制还不清楚。因此,揭示其潜在的分子机制对于改善PTC患者的病情、预后及术后生活质量至关重要。
miRNA是一种小分子RNA,约由25个核苷酸组成,在早期发育、脂肪代谢、细胞分化等生物学活动中发挥着重要作用[2]。最近研究证明,miRNA的异常表达与恶性肿瘤的发生发展有着密不可分的联系。在肝细胞癌[3-4]、乳腺癌[5-6]、结直肠癌[7]中作为抑癌基因或癌基因参与细胞的增殖、凋亡和侵袭,提示其在肿瘤进展的作用机制中具有重要的研究价值。Cong等[8]检测了肿瘤基因组图谱数据库中近500例PTC标本和60例正常甲状腺标本中miRNA的表达情况,研究显示与正常的甲状腺组织相比,PTC中miR-221表达上调。目前对miR-221作用机制及功能研究较少,本研究旨在探索miR-221的各种生物学行为,并探讨miR-221在PTC发生中可能的作用机制。
1 材料与方法
1.1 实验材料
本实验51对PTC组织及其癌旁正常组织均来自贵州医科大学附属医院。所有标本均在30 min内取材,取得标本后迅速放入液氮罐内冷藏,最后移至到-80 ℃的冰箱储存。所有标本均有完整的临床资料,术前均未进行化疗和放疗。PTC K1细胞系购自上海素尔生物科技有限公司,TRIzol试剂盒购自Ambion公司;HiScript Reverse Transcriptase(RNase H)、5×HiScript Buffer、50×ROX Reference Dye 2和SYBR Green Master Mix购自VAZYME公司;ddH2O(DNase/RNase Free)购自Genecopoeia公司;Ribonuclease Inhibitor、dNTP、Taq Plus DNA Polymerase和DL2000 DNA Marker购自TIANGEN公司;Random Primer(N6)购自AIDLAB公司;引物合成来自擎科公司。DMEM、胎牛血清、青-链霉素双抗、胰蛋白酶-EDTA(0.25%),酚红,No EDTA和Opti-MEM®购自Gibco公司;MTT购自Biosharp公司;lipofectamine 2000购自Invitrogen公司;周期检测盒购自凯基生物;APC/7-AAD凋亡试剂盒购自三箭。miR-221抑制物阴性对照序列:5'-CAG UAC UUU UGU UGA GUA CAA-3',miR-221抑制剂序列:5'-GAA ACC CAG CAG ACA AUG UAG CU-3'。
1.2 方法
1.2.1 细胞培养、细胞转染及分组 PTC K1细胞系用含10%胎牛血清的培养液,于37 ℃ 5% CO2饱和湿度条件下进行培养。取状态良好的处于对数生长期的K1细胞分成3组:空白对照、阴性对照序列转染组(阴性对照组)、miR-221抑制物转染组(miR-221抑制物),接种于96孔板,于37 ℃ 5% CO2培养箱中培养,待细胞生长达70%时,用Lipofectamine 2000分别转染miR-221抑制剂阴性对照和miR-221抑制剂,转染后继续培养相应时间。
1.2.2 qRT-PCR检测甲状腺组织miR-221的表达 按照TRIzol试剂盒说明书提取总RNA,按 照TransScript First-Strand cDNA Synthesis SuperMix for RT-PCR试剂盒说明书对其进行反转录合成cDNA。按照TransStart Green qPCR SuperMix试剂盒说明书进行PCR反应,引物序列如下:U6正向引物:5'-CGC TTC GGC AGC ACA TAT AC-3',U6反向引物:5'-AAA TAT GGA ACG CTT CAC GA-3',hsa-miR-221-3p环引物:5'-GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GAT ACG ACG AAA CCC A-3',hsa-miR-221-3p正向引物:5'-TGC GCA GCT ACA TTG TCT GCG G-3',反向引物:5'- CCA GTG CAG GGT CCG AGG TAT T-3'。PCR反应条件是:50 ℃ 2 min,95 ℃ 10 min;95 ℃ 30 s,60 ℃ 30 s,40个循环。每个样本重复3次,用ABI QuantStudio 6实时荧光定量PCR仪进行荧光定量PCR实验。最终数据以2-(△△ct)进行分析。
1.2.3 qRT-PCR检测K1细胞miR-221的表达 按照TRIzol试剂盒说明书从培养细胞中提取总RNA,然后按照TransScript First-Strand cDNA Synthesis SuperMix for RT-PCR试剂盒说明书对其进行反转录合成cDNA。按照TransStart Green qPCR SuperMix试剂盒说明书进行PCR反应,引物序列和PCR反应条件同上。每个样本重复3次,用ABI QuantStudio 6实时荧光定量PCR仪进行荧光定量PCR实验。最终数据以2-(△△ct)进行分析。
1.2.4 MTT比色法检测细胞增殖 取对数生长期,培育状态良好的K1细胞,接种于96孔板,同时设空白组,阴性对照组、miR-221抑制物组。37 ℃过夜,细胞培养所需时间后,每孔加入10 μL MTT,37 ℃培养4 h,吸出培养基,150 μL DMSO震荡10 min,酶标仪测定各孔吸光值OD568 nm。实验至少重复3次。
1.2.5 流式细胞仪分析细胞周期 分别取对数生长期,培育状态良好的空白对照组、NC转染组、miR-221 inhibitor转染组K1细胞,用0.25%胰酶消化细胞,终止后收集细胞,1 000 r/min,5 min,去上清,PBS重悬润洗2次,1 000 r/min,5 min,去上清,100 μL PBS重悬细胞,缓慢加入700 μL预冷的80%乙醇,使乙醇终浓度为70%,4 ℃固定4 h以上,1 000 r/min,5 min,预 冷PBS润洗2次,加入100 μL RNase(50 μg/mL),37 ℃孵育30 min,加入400 μL PI(50 μg/mL),4 ℃避光染色30 min,流式细胞仪检测。实验至少重复3次。
1.2.6 流式细胞仪分析细胞凋亡率 分别取对数生长期,培育状态良好的空白对照组、NC转染组、miR-221 inhibitor转染组K1细胞,培养所需时间后,进行收集,1 200 r/min,5 min离心,去上清,加PBS重悬,用PBS润洗2次,1 200 r/min,5 min,按照Annexin V-APC/7-AAD细胞凋亡检测试剂盒操作说明进行,加入500 μL结合缓冲液,重悬细胞,加5 μL Annexin V-APC混匀后加5 μL 7-AAD,混匀,室温避光反应5~15 min(同时设阴性对照,即正常细胞不加Annexin V-APC和7-AAD;以凋亡效果最明显的溶剂组作为阳性对照,设对照组 1和2,对照组1只加5 μL AnnexinV-APC单标;对照组2只加5 μL 7-AAD单标)。上机检测。实验至少重复3次。
1.2.7 Transwell小室检测细胞侵袭力 取处理好的 K1细胞,PBS清洗,0.25%胰酶消化收集,1 000 r/min,5 min离心,去上清,PBS清洗后,DMEM培养基重悬细胞,计数,DMEM培养基稀释细胞浓度至2×104/mL,备用,将 Matrigel在4 ℃提前1 d融化,Transwell小室、24孔培养板和枪头在-20 ℃过夜预冷,用无血清培养基稀释 Matrigel至终浓度1 mg/mL,冰上操作,在24孔板中注入4 ℃预冷800 μL 10% FBS DMEM培养基(含双抗),并放入Transwell小室,在Transwell小室上室底部中央垂直加入100 μL终浓度为 1 mg/mL的Matrigel,37 ℃室温温育成胶状后,在Transwell上室分别接入200 μL各组细胞悬液,37 ℃,5%CO2培养箱培养24 h,取出Transwell,PBS清洗小室,70%冰乙醇固定1 h,染色后,常温静置20 min,PBS清洁,用无污染的棉球将上室一侧的没有迁移的细胞擦净,显微镜下仔细观察并拍照。实验至少重复3次。
1.3 统计学处理
应用SPSS 20.0软件进行处理,所有数据都是以均数±标准差( ±s)方差表达。组间统计学显著性差异使用单因素方差分析或未配对独立样本 t检验。检验水准:α=0.05。
±s)方差表达。组间统计学显著性差异使用单因素方差分析或未配对独立样本 t检验。检验水准:α=0.05。
2 结 果
2.1 qRT-PCR检测hsa-miR-221-3p在PTC癌组织及癌旁组织中的表达
PCR结果显示,51例癌组织和癌旁组织的相对表达量分别为1.208 61±0.120 872,0.626 33±0.075 037,癌组织的相对表达量明显高于癌旁组织,差异有统计学意义(P<0.05)(图1)。
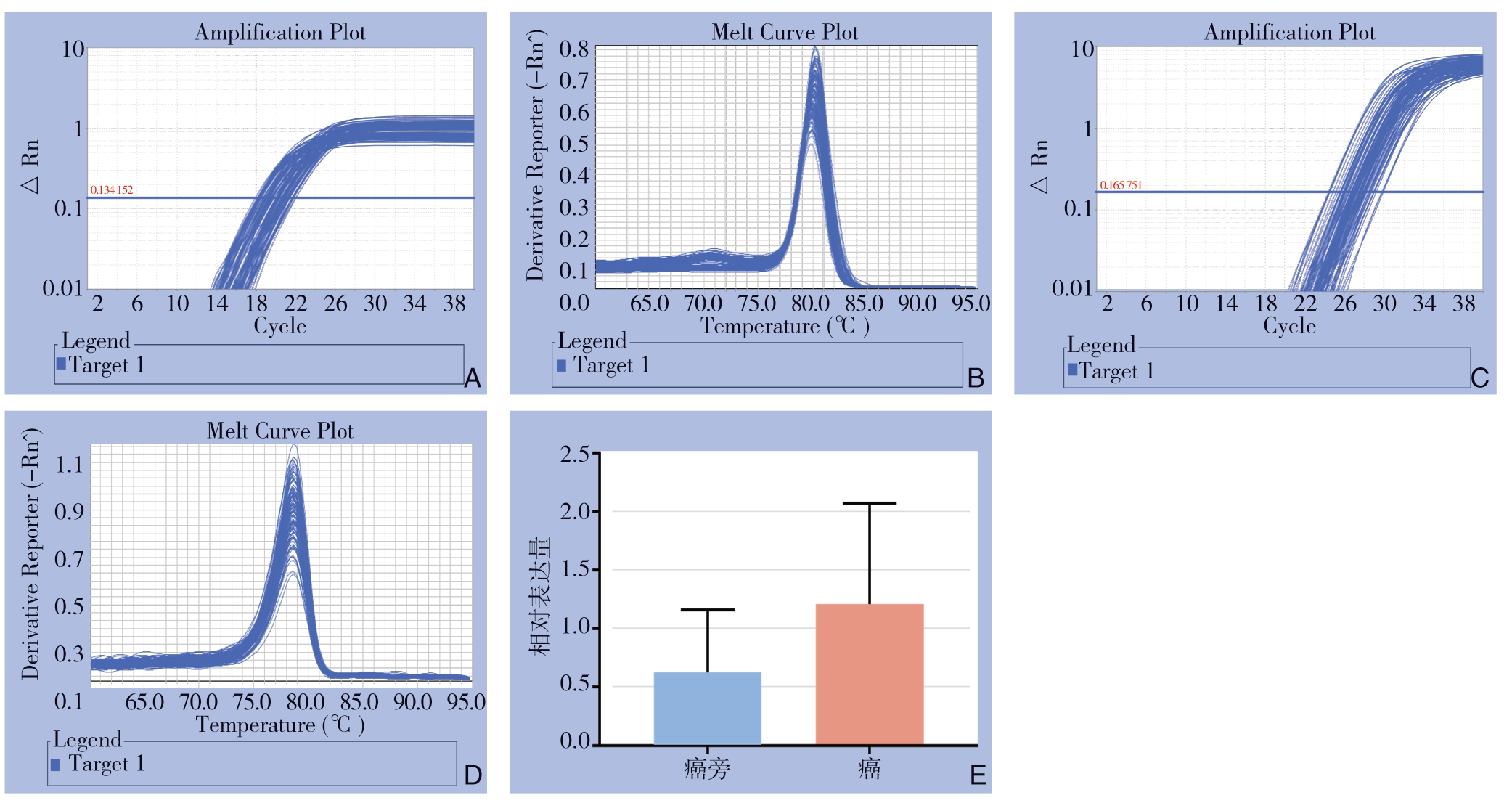
图1 qRT-PCR检测hsa-miR-221-3p的表达
Figure1 The expression of hsa-miR-221-3p was measured by qRT-PCR
A:U6扩增曲线;B:U6溶解曲线;C:hsa-miR-221-3p扩增曲线;D:hsa-miR-221-3p溶解曲线;E:hsa-miR-221-3p相对表达量
A:U6 amplification curve; B:U6 dissolution curve; C:hsa-miR-221-3p amplification curve; D:hsa-miR-221-3p dissolution curve; E:Relative expression levels of hsa-miR-221-3p
2.2 miR-221抑制物下调miR-221的效率
qRT-PCR结果显示,空白对照组、阴性对照组、miR-221抑制物组miR-221相对表达量平均值分别为0.997、0.984、0.277,miR-221抑制物组miR-221的表达量明显低于空白对照组与阴性对照组(均P<0.05),而后两组间差异无统计学意义(P>0.05)(图2)。
2.3 miR-221对PTC细胞增殖能力的影响
MTT实验结果显示在同一时间,miR-221抑制物组较其他组吸光值明显降低(均P<0.05),而阴性对照组与空白对照组间差异无统计学意义(P>0.05)(图3)。各组增殖率为:空白对照组100.00%、阴性对照组97.89%、miR-221抑制物组61.77%。
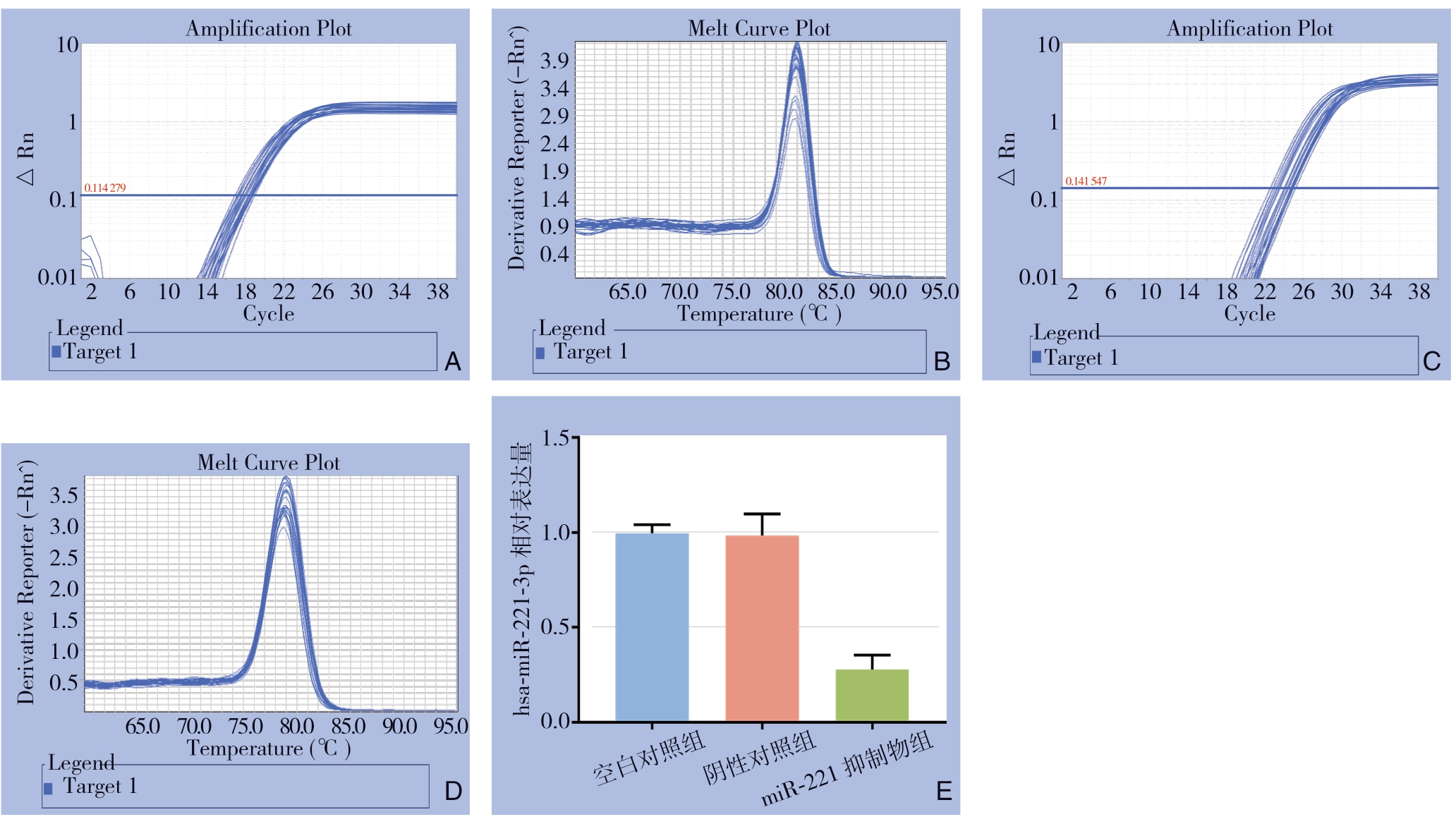
图2 miR-221 inhibitor的下调效率
Figure2 The downregulation efficiency of miR-221 inhibitor
A:U6扩增曲线;B:U6溶解曲线;C:hsa-miR-221-3p扩增曲线;D:hsa-miR-221-3p溶解曲线;E:hsa-miR-221-3p相对表达量
A:U6 amplification curve; B:U6 dissolution curve; C:hsa-miR-221-3p amplification curve; D:hsa-miR-221-3p dissolution curve; E:Relative expression levels of hsa-miR-221-3p
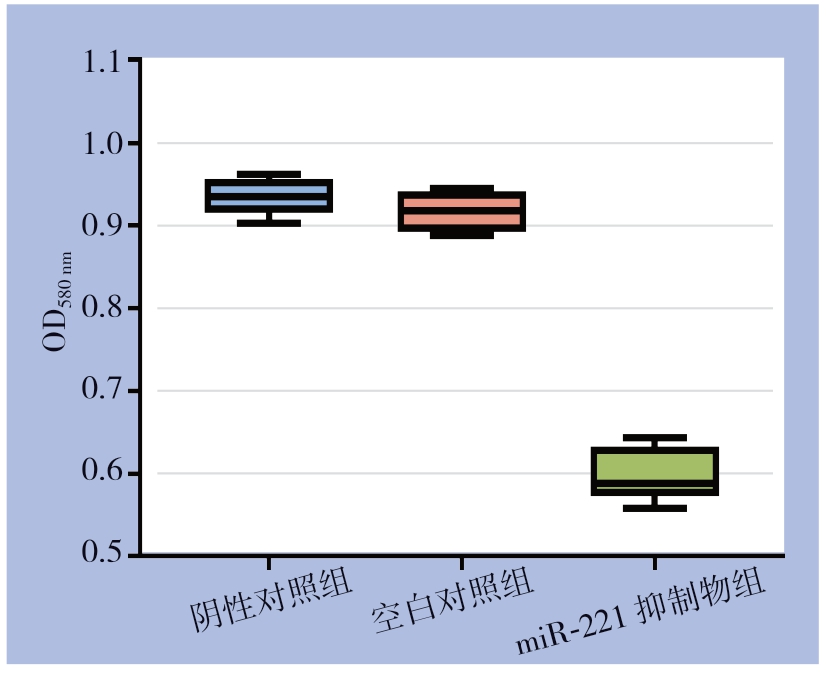
图3 miR-221对甲状腺乳头状癌细胞增殖的影响
Figure3 Effect of miR-221 on proliferation of papillary thyroid cancer cells
2.4 miR-221对PTC细胞凋亡和周期的影响
3次凋亡实验显示,空白对照组凋亡率分别为7.40%、6.58%、6.71%;阴性对照组凋亡率分别为6.79%、6.42%、6.72%;miR-221抑制物组凋亡率分别为23.43%、25.03%、24.77%,miR-221抑制物组较空白对照组与阴性对照组凋亡比例明显增加(均P<0.05),而阴性对照组与空白对照组的凋亡率无统计学差异(P>0.05)(图4)。细胞周期实验显示,miR-221抑制物组G0/G1期比例较空白对照组与阴性对照组转染组,而G2/M期的比例较空白对照组与阴性对照组转染组明显降低(均P<0.05),而阴性对照组与空白对照组的细胞周期分布无统计学差异(P>0.05)(图5)。
2.5 miR-221对PTC细胞侵袭能力的影响
侵袭实验中,每组设3个平行样本,每个样本观测5个视野(×200),空白对照组、阴性对照组、miR-221抑制物组的平均侵袭数为49.2、50.0、33.2,miR-221抑制物组较空白对照组与阴性对照组平均侵袭数明显减少(均P<0.05),而后两组间差异无统计学意义(图6)。
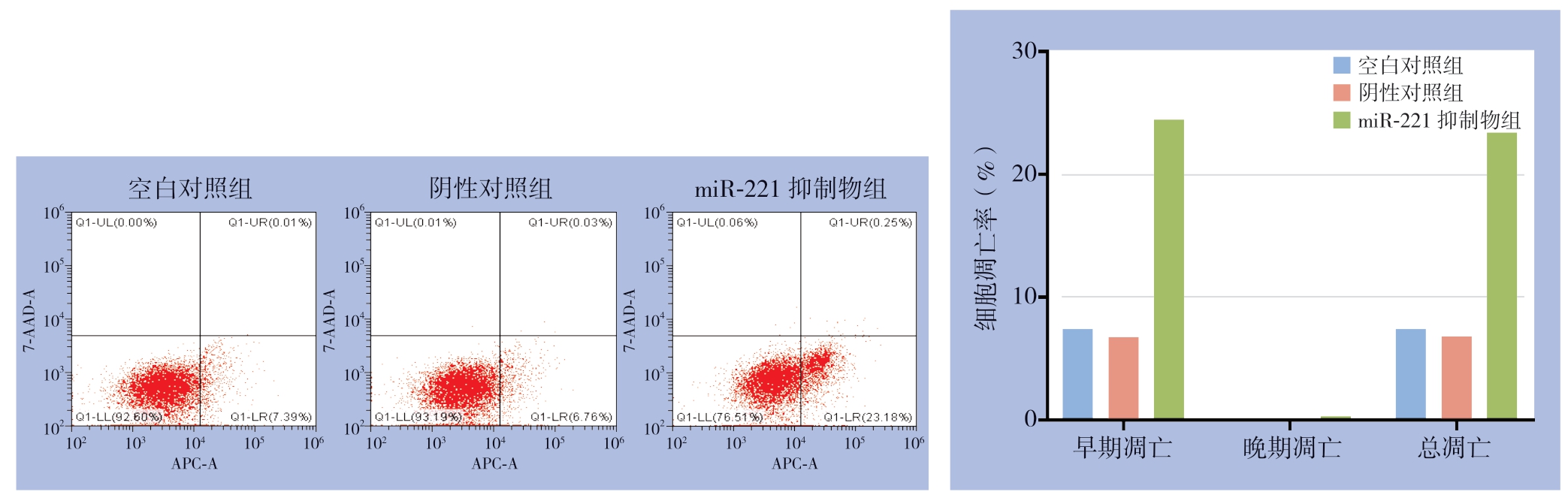
图4 miR-221对PTC细胞凋亡的影响
Figure4 Influence of miR-221 on apoptosis of PTC cells
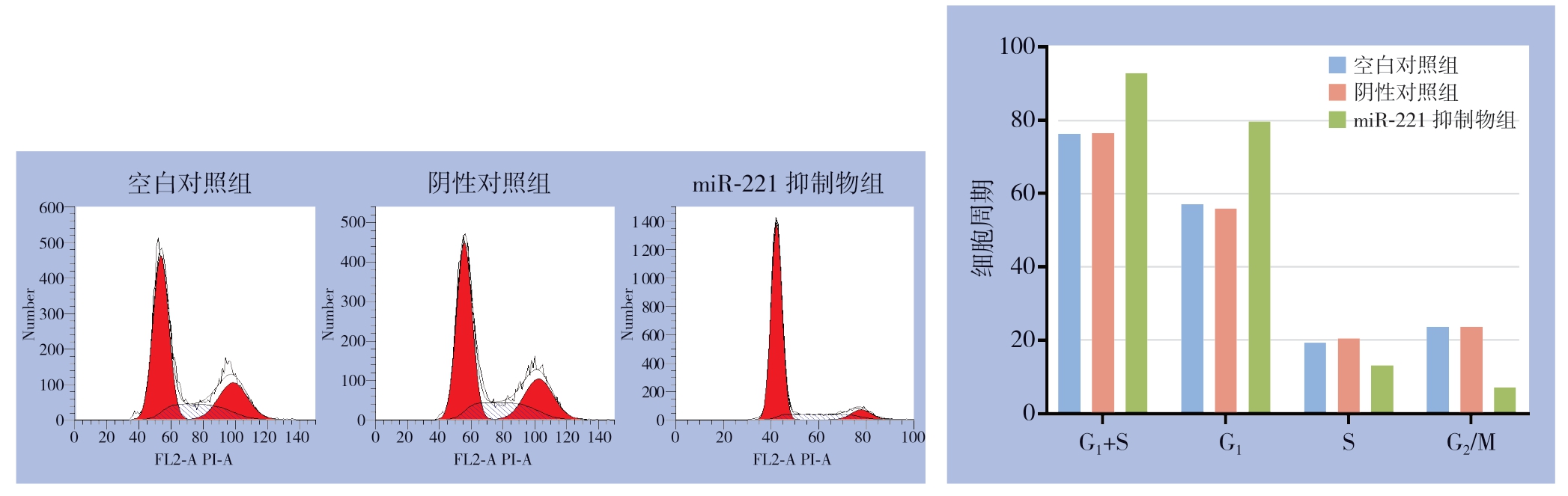
图5 miR-221对PTC细胞周期的影响
Figure5 Influence of miR-221 on cell cycle of PTC cells
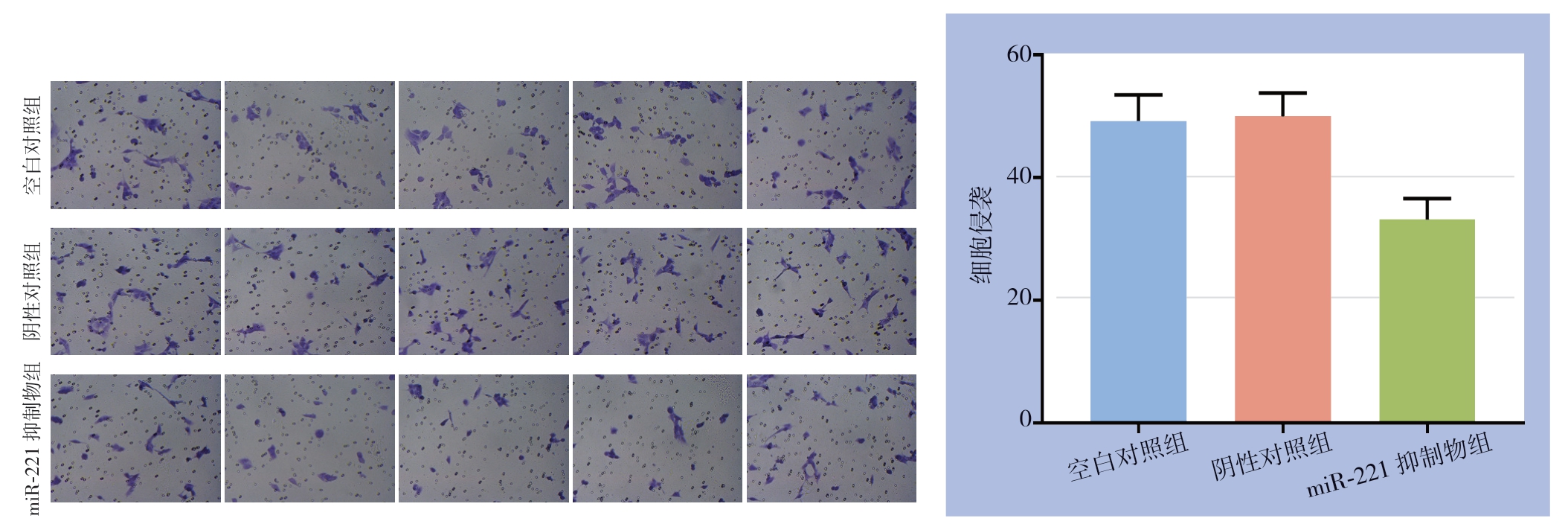
图6 miR-221对PTC细胞侵袭的影响
Figure6 Influence of miR-221 on the invasion ability of PTC cells
3 讨 论
miR-221在许多肿瘤中均表达出了致癌性。通过数据库检索显示,在成胶质细胞瘤[9]、肝细胞癌[10]、乳腺癌[11]、宫颈癌[12]、卵巢癌[13]、黑色素瘤[14]、甲状腺癌[15-16]、前列腺癌[17]中miR-221通过异常高表达促进了肿瘤细胞的恶性增殖、免疫逃逸、侵袭和转移。但是,在一些其他肿瘤中 miR-221也表达出了抑癌性。例如,miR-221通过靶向Ecm29,减弱了前列腺癌PCa细胞的迁移和侵袭[18]。miR-221通过针对红白血病细胞和胃肠道间质肿瘤中的Kit,从而抑制了癌细胞增殖并诱导了细胞凋亡[19]。此外,Okamoto等[20]发现miR-221在人胆管癌细胞中通过靶向调节亚基1(PIK3R1)的磷酸肌醇3激酶,从而抑制HuH28细胞增殖并赋予Gem敏感性。表明在不同肿瘤中,miR-221所起到的作用可能完全不同。
近些年来,通过对甲状腺癌的研究发现,不同病理类型的甲状腺癌发生与发展均和miRNA有着密切的关系。本研究发现,甲状腺乳头状癌组织及甲状腺乳头状癌细胞中miR-221表达明显上调,与Cerutti等[21]研究结果一致,认为PTC组织中miR-221的表达相比正常甲状腺组织显著增加。上述分析结果通过Northern印迹法和RT-PCR被进一步证实。此外,在甲状腺结节细针穿刺细胞学检查发现,miR-221过表达的甲状腺结节,经外科手术取活检后被最终确诊为PTC[21]。人类PTC来源的细胞系中通过阻断过表达的发现,miR-221的过度表达在PTC癌变中起关键作用。在FTC中,研究发现miR-197和miR-346表达的增加有助于FTC的发生,它可能通过干扰基因表达而起作用[22]。而miR-221虽然与PTC的致癌作用相关,但与FTC的发展无明显相关性[23]。在甲状腺未分化癌中,通过miRNA芯片微阵列分析ATC的miRNA发现,与正常甲状腺组织相比,miR-26a、miR-30d、miR-125b、miR-30a-5P的表达显著减少[24],而miR-221并未在ATC中上调[25]。在MTC中,miRNA的报道较为少见,仅有的报道中也并未显示miR-221和MTC的关系。目前的研究表明,miR-221作为一种致癌基因参与了甲状腺癌的发生和发展。
在PTC体外实验中,本研究发现miR-221较正常甲状腺组织明显高表达,通过向K1细胞系转染miR-221抑制物后,发现细胞增殖能力显著降低,细胞凋亡率显著升高,细胞生长周期明显延长,侵袭能力显著降低,表明miR-221促进了K1细胞的增殖和侵袭能力,降低了凋亡能力和细胞周期。
PI3K/Akt起始的细胞途径受到癌基因或抑癌基因的正向或负向控制,而这些基因又被增强或受多种因素(例如miRNA)抑制[26]。磷酸酶张力蛋白同源物(PTEN)在调节细胞黏附,增殖和迁移的信号通路中起到至关重要的作用。PTEN是包括肝癌在内的多种癌症的肿瘤抑制因子,他影响Akt和ERK信号通路[27]。据报道,在许多人类癌症中,miR-221通过下调PTEN来增强Akt的磷酸化[28]。 在胃癌细胞中,miR-221簇已显示出靶向肿瘤抑制基因PTEN,导致癌细胞增殖和放射抗性增加[29]。由于PTEN是miR-221的潜在靶标[30],因此miR-221可能和Akt通路的增强存在联系。因此笔者推测,miR-221对PTC增殖、凋亡、侵袭的影响可能与下调PTEN从而增强Akt信号通路有关。本研究存在着一定的欠缺和不足,虽然本研究探讨了miR-221在PTC发生和发展中的作用,但对于它产生作用的机制并没有做更进一步的实验研究,只能通过往期的报道来进行粗略的预测。
本研究发现miR-221在PTC组织及细胞中呈高表达;术前检测miR-221的表达状态对于提高甲状腺癌诊断率可能具有重要意义。抑制miR-221表达明显抑制PTC K1细胞株的增殖、促进细胞株的凋亡和降低细胞株的侵袭性。为侵袭性PTC的靶基因治疗提供了一定的理论依据。
[1] Kim SJ,Myong JP,Jee HG,et al.Combined effect of Hashimoto's thyroiditis and BRAF(V600E) mutation status on aggressiveness in papillary thyroid cancer[J].Head Neck,2016,38(1):95-101.doi:10.1002/hed.23854.
[2] Rupaimoole R,Slack FJ.MicroRNA therapeutics:towards a new era for the management of cancer and other diseases[J].Nat Rev Drug Discov,2017,16(3):203-222.doi:10.1038/nrd.2016.246.
[3] 赵新阳,肖朝文,郑小林,等.miR-96的表达对肝细胞癌细胞迁移和侵袭的影响[J].中国普通外科杂志,2017,26(7):877-882.doi:10.3978/j.issn.1005-6947.2017.07.010.
Zhao XY,Xiao CW,Zheng XL,et al.Influence of miR-96 expression on migration and invasion of hepatocellular carcinoma cells[J].Chinese Journal of General Surgery,2017,26(7):877-882.doi:10.3978/j.issn.1005-6947.2017.07.010.
[4] 秦麒麟,李清龙.miRNA-122与肝细胞癌的研究进展[J].中国普通外科杂志,2015,24(1):105-109.doi:10.3978/j.issn.1005-6947.2015.01.020.
Qin QL,Li QL.Research progress of miRNA-122 and hepatocellular carcinoma[J].Chinese Journal of General Surgery,2015,24(1):105-109.doi:10.3978/j.issn.1005-6947.2015.01.020.
[5] 徐泰.miRNA-639在乳腺癌中表达及其意义[J].中国普通外科杂志,2014,23(11):1506-1511.doi:10.7659/j.issn.1005-6947.2014.11.010.
Xu T.miRNA-639 expression in breast cancer and its significance[J].Chinese Journal of General Surgery,2014,23(11):1506-1511.doi:10.7659/j.issn.1005-6947.2014.11.010.
[6] 阮永威,田兴松,侯连泽.乳腺癌 p16,p53 基因蛋白和 mRNA 的表达及其意义[J].中國普通外科杂志,2008,17(5):497-501.
Ruan YW,Tian XS,Hou LZ.The significance of p16 and p53 gene protein and mRNA expression on breast cancer[J].Chinese Journal of General Surgery,2008,17(5):497-501.
[7] 于卫芳,翟从劼,樊智彬,等.miR-150 和 miR-134 在结直肠癌及腺瘤中的表达[J].中国普通外科杂志,2014,23(10):1349-1354.doi:10.7659/j.issn.1005-6947.2014.10.009.
Yu WF,Zhai CJ,Fan ZB,et al.Expression of miR-150 and miR-134 in colorectal cancer and colorectal adenoma[J].Chinese Journal of General Surgery,2014,23(10):1349-1354.doi:10.7659/j.issn.1005-6947.2014.10.009.
[8] Cong D,He M,Chen S,et al.Expression profiles of pivotal microRNAs and targets in thyroid papillary carcinoma:an analysis of The Cancer Genome Atlas[J].Onco Targets Ther,2015,8:2271-2277.doi:10.2147/OTT.S85753.
[9] Zhang CZ,Zhang JX,Zhang AL,et al.miR-221 and miR-222 target PUMA to induce cell survival in glioblastoma[J].Mol Cancer,2010,9:229.doi:10.1186/1476-4598-9-229.
[10] Fornari F,Gramantieri L,Ferracin M,et al.miR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma[J].Oncogene,2008,27(43):5651-5661.doi:10.1038/onc.2008.178.
[11] Zhao JJ,Lin JH,Yang H,et al.MicroRNA-221/222 Negatively regulates estrogen receptor alpha and is associated with tamoxifen resistance in breast cancer[J].J Biol Chem,2008,283(45):31079-31086.doi:10.1074/jbc.M806041200.
[12] Zhou CF,Ma J,Huang L,et al.Cervical squamous cell carcinomasecreted exosomal miR-221-3p promotes lymphangiogenesis and lymphatic metastasis by targeting VASH1[J].Oncogene,2019,38(8):1256-1268.doi:10.1038/s41388-018-0511-x.
[13] Wurz K,Garcia RL,Goff BA,et al.miR-221 and miR-222 alterations in sporadic ovarian carcinoma:relationship 12 Journal of Oncology to CDKN1B,CDKNIC and overall survival[J].Genes Chromosomes Cancer,2010,49(7):577-584.doi:10.1002/gcc.20768.
[14] Felicetti F,Errico MC,Botteroetal L,et al.The Promyelocytic Leukemia Zinc finger-microRNA-221/-222 Pathway Controls Melanoma Progression Through Multiple Oncogenic Mechanisms[J].Cancer Res,2008,68(8):2745-2754.doi:10.1158/0008-5472.CAN-07-2538.
[15] Mardente S,Mari E,Consorti F,et al.HMGB1 induces the overexpression of miR-222 and miR-221 and increases growth and motility in papillary thyroid cancer cells[J].Oncol Rep,2012,28(6):2285-2289.doi:10.3892/or.2012.2058.
[16] Mardente S,Mari E,Massimi I,et al.HMGB1-Induced cross talk between PTEN and miRs 221/222 in thyroid cancer[J].Biomed Res Int,2015,2015:512027.doi:10.1155/2015/512027.
[17] Galardi S,Mercatelli N,Giorda E,et al.miR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1[J].J Biol Chem,2007,282(32):23716-23724.doi:10.1074/jbc.M701805200.
[18] Goto Y,Kojima S,Nishikawa R,et al.MicroRNA expression signature of castration-resistant prostate cancer:the microRNA-221/222 cluster functions as a tumour suppressor and disease progression marker[J].Br J Cancer,2015,113(7):1055-1065.doi:10.1038/bjc.2015.300.
[19] Gits CMM,van Kuijk PF,Jonkers MBE,et al.MiR-17-92 and miR-221/222 cluster members target KIT and ETV1 in human gastrointestinal stromal tumours[J].Br J Cancer,2013,109(6):1625-1635.doi:10.1038/bjc.2013.483.
[20] Okamoto K,MiyoshiK,Murawaki Y.miR-29b,miR-205 and miR-221 enhance chemosensitivity to gemcitabine in HuH28 human cholangiocarcinoma cells[J].PLoS One,2013,8(10):e77623.doi:10.1371/journal.pone.0077623.
[21] Cerutti J,Trapasso F,Battaglia C,et al.Block of c-myc expression by antisense oligo nucleotides inhibits proliferation of human thyroid carcinoma cell lines[J].Clin Cancer Res,1996,2(1):119-126.
[22] He H,Jazdzewski K,Li W,et al.The role of microRNA genes in papillary thyroid carcinoma[J].Proc Natl Acad Sci U S A,2005,102(52):19075-19080.doi:10.1073/pnas.0509603102.
[23] Visone R,Pallante P,Vecchione A,et al.Specific microRNAs are downregulated in human thyroid anaplastic carcinomas[J].Oncogene,2007,26(54):7590-7595.doi:10.1038/sj.onc.1210564.
[24] Liu CG,Calin GA,Meloon B,et al.An oligonucleotide microchip for genome-wide microRNA pro ling in human and mouse tissues[J].Proc Natl Acad Sci U S A,2004,101(26):9740-9744.doi:10.1073/pnas.0403293101.
[25] Pallante P,Visone R,Ferracin M,et al.MicroRNA deregulationin human thyroid papillary carcinomas[J].Endocr Related Cancer,2006,13(2):497-508.doi:10.1677/erc.1.01209.
[26] Touré F,Zahm JM,Garnotel R,et al.Receptor for advanced glycation end-products (RAGE) modulates neutrophil adhesion and migration on glycoxidated extracellular matrix[J].Biochem J,2008,416(2):255-261.doi:10.1042/BJ20080054.
[27] Liu LT,Chang HC,Chiang LC,et al.Histone deacetylase inhibitor up-regulates RECK to inhibit MMP-2 activation and cancer cell invasion[J].Cancer Res,2003,63(12):3069-3072.
[28] Muniyan S,Ingersoll MA,.Batra SJ,et al.Cellular prostatic acid phosphatase,a PTEN-functional homologue in prostate epithelia,functions as a prostate-specific tumor suppressor[J].Biochim Biophys Acta,2014,1846(1):88-98.doi:10.1016/j.bbcan.2014.04.006.
[29] Zhang CZ,Han L,Zhang AL,et al.MicroRNA-221 and microRNA-222 regulate gastric carcinoma cell proliferation and radioresistance by targeting PTEN[J].BMC Cancer,2010,10:367.doi:10.1186/1471-2407-10-367.
[30] Meng F,Henson R,Lang M,et al.Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines[J].Gastroenterology,2006,130(7):2113-2129.doi:10.1053/j.gastro.2006.02.057.
