脾动脉瘤(splen ic artery aneurysm,SAA)是指脾动脉的非正常扩张、直径超过1 cm的一类内脏动脉瘤疾病;可发生于脾动脉主干,亦可发生于脾动脉分支。SAA可分为真性动脉瘤和假性动脉瘤,其中60%为真性动脉瘤[1]。目前治疗SAA的唯一方法为外科手术。既往SAA的手术方式为切除动脉瘤,创伤较大,且绝大部分需同时切除脾脏。目前随之介入技术的发展,越来越多的SAA可采取介入腔内技术进行治疗。介入技术在极小侵入操作和保留靶器官的情况下即可完成手术,其具体方式、疗效及预后取决于瘤体的直径、位置及形状[2]。本文回顾了2012—2019年湘雅医院血管外科收治的30例SAA患者资料。所有患者均为真性动脉瘤,全部采用介入手术并保留脾脏;肯定了SAA腔内治疗的有效性、安全性及微创性。
1 资料与方法
1.1 临床资料
收集湘雅医院血管外科2012年1月—2019年12月入院的30例SAA患者的资料,均为位于脾动脉主干的单发真性动脉瘤,直径≥2 cm,共30 个瘤体。其中男10例,女20例;年龄14~71岁,中位年龄52.1岁。其中伴有左上腹痛症状的有7例,余均无自觉症状。合并肝硬门脉高压的11例,合并多次妊娠史的6例,合并高血压的4例,合并动脉硬化闭塞症的2例,无明显诱因的7例(表1)。
表1 30 例SAA 患者的临床资料[n(%)]
Table1 Basic clinical data of all 30 patients with SAA [n(%)]
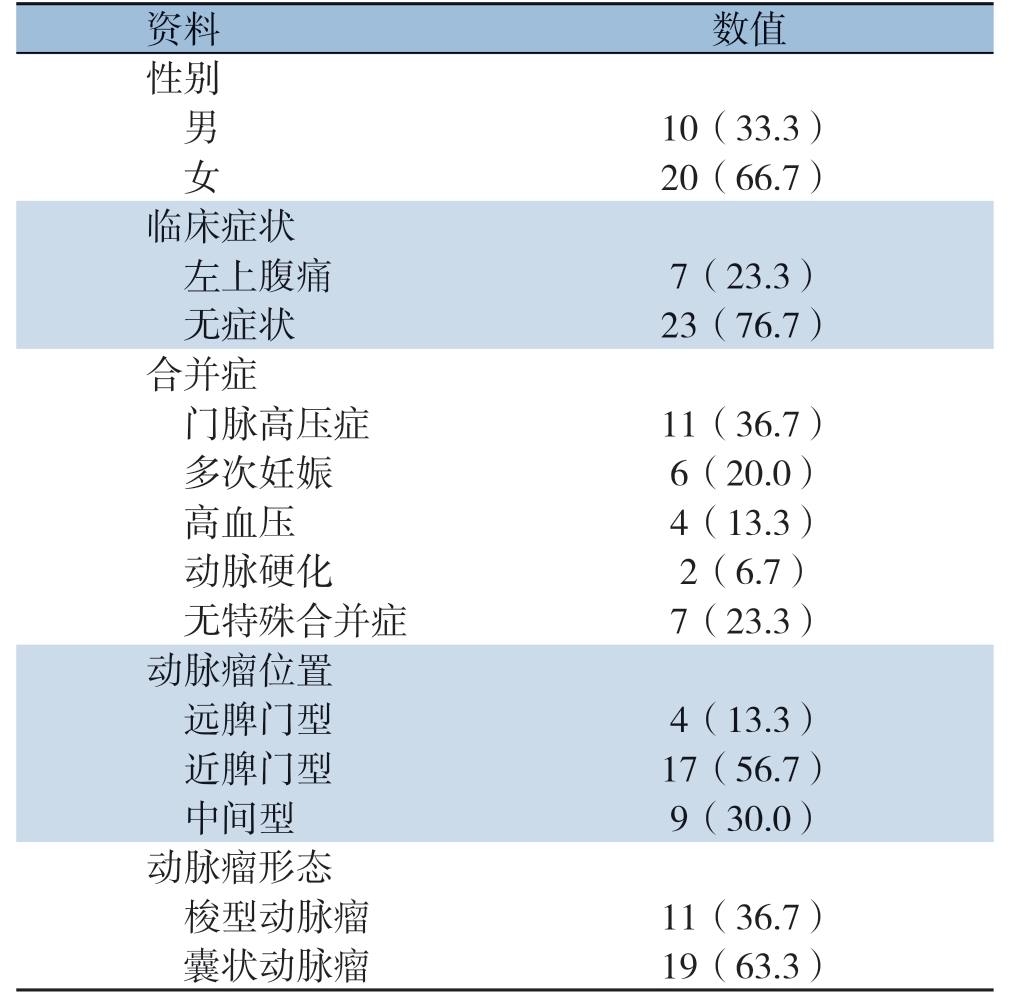
资料 数值性别 男 10(33.3)女 20(66.7)临床症状 左上腹痛 7(23.3)无症状 23(76.7)合并症 门脉高压症 11(36.7)多次妊娠 6(20.0)高血压 4(13.3)动脉硬化 2(6.7)无特殊合并症 7(23.3)动脉瘤位置 远脾门型 4(13.3)近脾门型 17(56.7)中间型 9(30.0)动脉瘤形态 梭型动脉瘤 11(36.7)囊状动脉瘤 19(63.3)
1.2 影像学检查
所有患者均行腹部CTA确诊动脉瘤,且瘤体最大直径≥2 cm。根据瘤体发生部分可分为:(1)远脾门型,4例(图1A);(2)中间型,9例(图1B);(3)近脾门型,17例(图1C)。根据瘤体形态可分为:(1)梭形动脉瘤:11例;(2)囊状动脉瘤:19例。

图1 腹主动脉CTA 示3 种类型的SAA A:远脾门型SAA;B:中间型SAA;C:近脾门型SAA
Figure 1 Three types of SAA shown by aortic abdominal CTA A:Distal splenic artery aneurysm;B:Middle splenic artery aneurysm;C:Proximal splenic artery aneurysm
1.3 手术方法
腔内手术均采用局部麻醉。常规右侧股动脉入路,置入6 F Guiding长鞘,导管配合导丝选入腹腔干及脾动脉内。处理SAA最常用的有以下3种方法。(1)覆膜支架腔内隔绝术。适用于动脉瘤位于主干血管,且距离脾动脉开口处有足够长的距离,以保证支架有足够的锚定区。术前需仔细测量瘤体两端正常动脉数据。瘤体近端正常动脉应至少留有1 cm长距离作为锚定区,且避免合并分支动脉;支架直径根据近端动脉直径放大10%,如此可达到理想的隔绝效果。我院使用的是Vibahn覆膜支架(美国Gore公司),支架需完全覆盖病变段血管,且两端超过动脉瘤近远端边界至少1 cm。为减少支架术后内漏风险,可在路图模式下调整支架位置以达到准确释放。最终造影确认瘤体隔绝效果(图2)。(2)裸支架+弹簧圈栓塞术。若患者瘤体两端动脉支架锚定区合并有重要血管分支,覆膜支架可能覆盖分支动脉,可采取裸支架+弹簧圈封堵瘤体。裸支架在保留分支动脉血流通过的同时阻挡弹簧圈脱落导致其他重要动脉栓塞。首选导入裸支架(EverFlex,美国Cook公司)释放覆盖瘤体,然后导管经裸支架网眼选入瘤体内,送入栓塞弹簧圈填满瘤体(图3)。(3)弹簧圈或栓塞剂栓塞术。适用于瘤体位于脾动脉主干中远端、支架无法送达的动脉瘤。囊状动脉瘤:导管导丝选入脾动脉后,导丝配合导管选入瘤体内,经导管送入弹簧圈;若瘤体过大(>4 cm),可选用直径较大的电解脱弹簧圈,配合小弹簧圈及非黏附性液体栓塞剂,填满整个瘤体以达到栓塞效果;若载瘤动脉为分支动脉,需使用0.018微导丝配合微导管选入瘤体内栓塞瘤体;脾动脉中远端的梭形动脉瘤:导管导丝先选入瘤体远端的动脉内,使用弹簧圈栓塞流出道,再将导管退回瘤体近端的动脉内,栓塞流入道,以达到完全封闭瘤体的效果(图4)。介入手术完成后拔除导管导丝后,采用Perclose Proglide血管缝合器(美国Abbott公司)关闭股动脉穿刺口。
1.4 术后处理及随访
术后观察患者有无腹痛、呕吐、发热、穿刺点出血。对于行栓塞术患者,常规预防性使用抗生素;对于行支架置入术患者,术后常规口服阿司匹林0.1 g1次/d或氯吡格雷75 mg1次/d3个月;对于单纯行栓塞但未行支架置入术患者,未口服抗凝或抗血小板药物。术后卧床24 h后即可下床活动。术后3、6个月对患者行CTA检查随访。
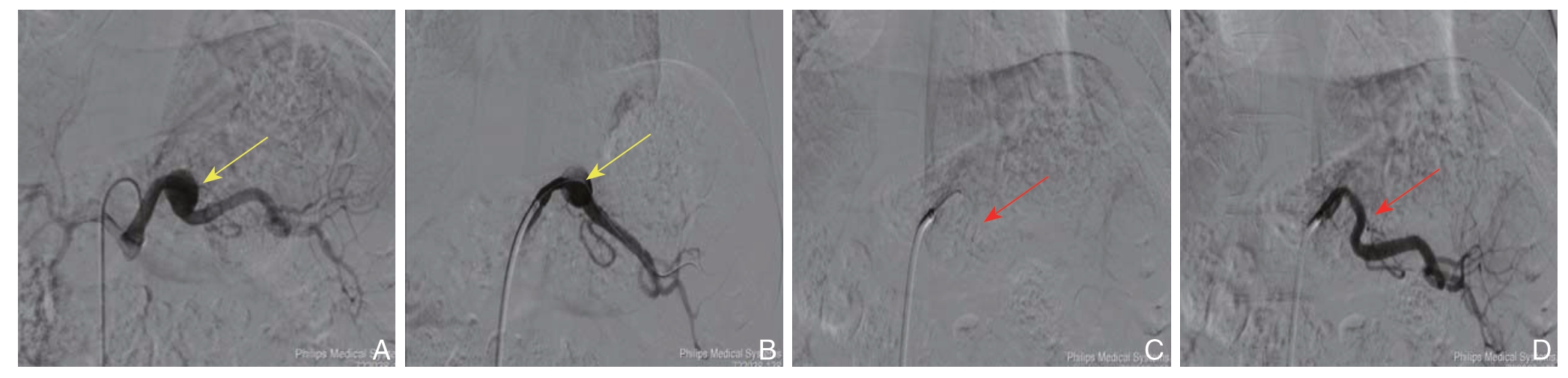
图2 DSA 示SAA 覆膜支架腔内隔绝术(黄色箭头显示SAA 瘤体,红色箭头显示覆膜支架)A:导管选入腹腔干,造影显示SAA 位置及形态;B:导丝选入SAA 远端;C:置入覆膜支架,支架覆盖瘤体并保证近、远端有足够锚定区;D:最后造影显示瘤体隔绝良好,脾动脉血流通畅
Figure 2 DSA demonstrating the endovascular exclusion with covered stent for SAA(the yellow arrow showing the SAA,the red arrow showing the covered stent)A:Catheter insertion into the splenic artery,and angiography showing the position and shape of the SAA;B:Guide wire introduced through the SAA to the distal end;C:Covered stent placement for excluding the aneurysm and ensuring the adequate proximal and distal landing zones;D:Angiography showing the successfully excluded aneurysm and patent splenic artery

图3 DSA 示裸支架+弹簧圈治疗SAA(红色箭头显示裸支架;绿色箭头显示弹簧圈)A:导管导丝选入载瘤动脉;B:置入裸支架覆盖瘤体;C:导管经裸支架选入瘤体内;D:经导管送入弹簧圈完全填塞瘤体
Figure 3 DSA demonstrating bared stent implantation with coil embolization for treatment of SAA(the red arrow showing the bare stent,the green arrow showing the coil)A:Insertion of catheter and guide wire into the parent artery;B:Coverage of the aneurysm with bared stent;C:Catheter introduced into the aneurysm through bare stent mesh;D:Stuffing the aneurysm with coil through catheter

图4 DSA 示SAA 栓塞术(黄色箭头显示SAA 瘤体,绿色箭头显示弹簧圈及栓塞剂)A:SAA 为瘤颈开口小的囊状动脉瘤;B:导管选入囊状动脉瘤,使用弹簧圈填满整个瘤体;C:SAA 为梭形动脉瘤;D:使用弹簧圈及栓塞剂填满瘤体,同时栓塞瘤体的输入动脉及输出动脉
Figure 4 DSA demonstrating coil embolization of the SAA(the yellow arrow showing the SAA,the green arrow showing the coil and embolization agent)A:A saccular aneurysm with narrow neck;B:Catheter introduced into the saccular aneurysm and stuffing the aneurysm with coil;C:A fusiform aneurysm;D:Stuffing the fusiform aneurysm with coil and embolization agent,and simultaneous embolization of the infl ow and outfl ow arteries of the aneurysm
2 结 果
2.1 手术结果
30 例患者均接受腔内手术治疗。瘤体直径21 ~52 m m。行覆膜支架置入术6 例(远脾门型3例,中间型3例),行动脉瘤栓塞术21例(近脾门型16例,中间型5例),行裸支架+弹簧圈栓塞术3例(远脾门型1例,中间型2例)。手术平均时间60.3 min。术中平均出血10.6 mL。
2.2 住院期间并发症及处理
术后出现栓塞综合征10例,症状为左上腹痛/呕吐/发热,平均体温37.8℃。症状出现时间从手术当天或术后第1 天,抽血查血常规、血生化指标,发现白细胞/转氨酶/淀粉酶异常5例;对于出现栓塞综合征患者采取对症处理如止痛、退热,未采取抗生素治疗;若症状持续24 h,加用激素(250 mL生理盐水+10 mg地塞米松静脉滴注1次/d)。上述症状持续时间未超过3 d,血化验指标在术后第4或第5天恢复正常。
术后1例出现右侧股动脉穿刺点出血,原因为患者过早下床,未达到足够制动时间(24 h)。采取右侧腹股沟八字绷带加压包扎,并嘱患者右下肢制动,24 h后股动脉出血停止,复查股动脉彩超未见假性动脉瘤形成,康复出院。
2.3 随访
随访时间3 ~12 个月,生存患者C T 复查示动脉瘤3 个月后已完全血栓化,未见瘤体增大、再通(图5)。复查期间,5例CT显示局灶性脾梗死(梗死范围<25%),均为弹簧圈或栓塞剂(伴或不伴裸支架置入)行栓塞术患者,但无临床症状,弹簧圈或栓塞剂未见移位(图6)。行覆膜支架术后患者复查未见脾梗死,支架内血流通畅,未见闭塞或血栓形成。

图5 SAA 术前及覆膜支架置入术后6 个月腹主动脉CTA(黄色箭头显示SAA 瘤体,红色箭头显示覆膜支架)A:术前SAA 为中间型梭形动脉瘤;B:横断面瘤体充满造影剂;C:6 个月后示瘤体隔绝良好,且脾动脉通畅;D:横断面示瘤体完全血栓化,未见造影剂流入,脾脏未见梗死
Figure 5 Abdominal aortic CTA images of SAA before operation and 6 month after covered stent implantation(the yellow arrow showing the SAA,the red arrow showing the covered stent)A:A fusiform aneurysm in the middle segment of the splenic artery before operation;B:Cross section of the aneurysm full of contrast material;C:Complete exclusion of the aneurysm and patent splenic artery on 6 months after operation;D:Complete occlusion of the aneurysm by thrombosis without endoleak of contrast agent,and no presence of splenic infarction

图6 SAA 栓塞术后或裸支架+弹簧圈术后6 个月腹主动脉CTA(黄色箭头显示SAA 瘤体,红色箭头显示裸支架,绿色箭头显示弹簧圈)A:SAA 为近脾门型囊状动脉瘤;B:瘤体内充满弹簧圈未见造影剂流入;C:SAA 为中间型梭形动脉瘤;D:瘤体内弹簧圈无移位或脱落,未见造影剂流入,裸支架内血流通畅,脾脏未见梗死
Figure 6 Abdominal aortic CTA images of SAA on 6 months after coil embolization with or without bare stent implantation(the yellow arrow showing the aneurysm,the red arrow showing the bare stent,the green arrow showing the coil)A:A saccular aneurysm in the proximal splenic artery;B:The aneurysm filled by the coil without endoleak of contrast agent;C:A fusiform aneurysm in the middle splenic artery;D:No displacement or detachment of the coil,no endoleak of contrast agent,patent flow through the bare stent,and no presence of splenic infarction
3 讨 论
SAA是发病率第三的动脉瘤疾病,仅次于腹主动脉瘤和髂动脉瘤;同时是发病率第一的内脏动脉瘤疾病,人群中的总体发病率约为0.8%[3],女性发病率明显高于男性[4],好发年龄为52~61岁[5]。SAA的诊断标准为脾动脉直径超过1 cm[6]。若脾动脉直径≥2 cm,则考虑有较高自发性破裂可能[7]。若脾动脉直径超过5 cm,则考虑为巨大SAA,有极高破裂大出血风险及病死率[8]。SAA根据发生部位可分为:(1)近脾门型:瘤体位于脾动脉远端1/3处,靠近脾脏,距脾动脉分叉处<5 cm,占总数的74%~87%;(2)远脾门型:瘤体位于脾动脉近端1/3处,距离脾脏>5 cm,占总数的6%;(3)中间型:瘤体位于脾动脉中段1/3处,占总数的20%~22%[9]。SAA根据瘤体形态可分为:(1)梭形动脉瘤,瘤体向动脉四周均匀扩张,成球形或纺锤形,占总数的25%;(2)囊状动脉瘤,瘤体向动脉一侧偏心性扩张,成烧瓶形或口袋状,占总数的75%[10]。SAA目前病因尚不明确,目前公认的导致脾动脉瘤样扩张的两大基本病理生理改变为:动脉壁病变和动脉内血压升高[11]。其他文献综述总结的SAA的危险因素有:(1)肝硬化,门脉高压[12];(2)高血压病[13];(3)动脉硬化[14];(4)糖尿病[11];(5)吸烟史[13];(6)胶原病[11];(7)女性[14];(8)抗胰蛋白酶缺乏症[15];(9)感染或炎症反应[12];(10)多次妊娠[16]。
SAA的主要危害来自于瘤体的破裂大出血导致的失血性休克,特别是对于直径>2 cm的S AA瘤体[17]。2%~10%的SAA患者会发生自发性破裂,但破裂后的SAA病死率可达到10%~40%,致死率极高[18]。然而,临床上多数SAA是在患者体检时无意中检出的,绝大部分SAA患者在瘤体破裂前无自觉症状或体征,这更增加了SAA的致命风险[19]。破裂型SAA的典型症状是急性发作的上腹部或左上腹部疼痛,可放射至左肩部;同时伴随症状有恶心、呕吐、低血压甚至休克[11]。SAA破裂后,血流可流入腹腔,引起腹腔内积血;或可破入胃肠道导致呕血、黑边、便血等消化道出血症状;一部分SAA可形成脾动脉-脾静脉瘘,引起肠系膜窃血综合征,导致肠梗阻等小肠缺血症状[20]。破裂型SAA患者中有20%~25%的病例,出血可首先被周边小网膜囊或血凝块包裹形成血肿,限制了进一步出血。这个时期患者主要表现为腹痛,但血流动力学可暂时维持稳定。当血肿内压力逐渐增大,最终仍会导致网膜囊破裂,血肿破入腹腔内,引起患者突发性休克[19]。
对于无症状的SAA患者,手术指征[21]是:(1)瘤体直径≥2 cm;(2)直径<2 cm,但进行性增大(增加直径>1 cm/年)的瘤体;(3)瘤体直径<2 cm,但合并妊娠或准备怀孕的女性患者,或合并肝硬化、门脉高压的患者。对于有明显腹痛或瘤体直径>5 cm的SAA患者,应尽快进行手术处理。对于已破裂的SAA患者,应急诊手术干预[22]。目前治疗SAA的主要手术方式为开放手术和介入腔内手术。在2010年以前我科治疗SAA以传统开放手术为主,应用最广泛的术式为脾动脉结扎+脾脏切除术,只有极少部分患者采取了保留脾脏的SAA切除并人工血管置换术。2010年以后我科SAA腔内治疗所占的比例逐年显著提高。以下介绍我科最常用的3种腔内治疗方法。(1)栓塞:栓塞是指在血管内人为地置入器械或药剂,诱导血管腔内血栓形成,改变血流动力学来治疗动脉瘤[23]。弹簧圈是使用最广泛也是最简单的栓塞材料[24]。对于梭形SAA,由于弹簧圈在瘤体内无法停留固定,所以弹簧圈不能用于填塞瘤体本身,而是用于堵塞瘤体两端的动脉以及其他可能导致返流的侧支血管。因为脾动脉远端存在胃网膜左动脉、胃短动脉以及其他丰富侧支血管代偿,因此即使载瘤动脉为脾动脉主干,彻底栓塞瘤体两侧动脉也不会导致脾梗死。相反靠近脾脏的梭形SAA行弹簧圈栓塞导致的脾脏缺血风险要高于远脾门型和中间型SAA[25]。另外,脾动脉主干的弹簧圈栓塞术可能发生弹簧圈、斑块或血栓脱落移位,堵塞远端动脉,导致靶器官缺血梗死[26]。对于囊状SAA,若瘤颈细窄,可将导管留置于瘤颈部,往瘤体内填入弹簧圈直到占满大部分体积,诱导瘤体内血栓化。窄瘤颈本身可以限制弹簧圈脱落到瘤体之外[23]。对于位置在脾动脉远端的SAA,常使用可解脱弹簧圈进行栓塞。此种弹簧圈在推送过程中通过固定装置连接于导丝上,在弹簧圈到达理想的栓塞位置,且成袢形态良好后,再用导丝解开连接装置使弹簧圈松脱;若弹簧圈无法到达栓塞位置,或到位置后无法成袢,通过导丝可回收弹簧圈。可解脱弹簧圈在降低弹簧圈脱落、异位栓塞风险的同时,能够达到更加精确的栓塞效果[23]。液体栓塞剂通常配合弹簧圈一起使用。栓塞剂本身为液体状,在接触血液成分后可迅速固体化,并可以诱导血栓形成。在使用弹簧圈占据大部分瘤体形成“骨架”后,再往瘤体内浇筑液体栓塞剂,填充骨架之间的空隙,达到SAA“完全栓塞”[27]。(2)覆膜支架:覆膜支架置入术是在动脉腔内导入覆膜的金属支架,来阻止主干动脉的血流进入瘤体内,达到隔绝动脉瘤的效果。覆膜支架置入术适合于位于脾动脉近端和中段的梭形动脉瘤或囊状动脉瘤。对于囊状动脉瘤,覆膜支架只需封堵瘤颈部;而对于梭形动脉瘤,覆膜支架需覆盖整个瘤体以及足够长距离的输入、输出动脉。(3)裸支架+栓塞:对于位于脾动脉近端的梭形动脉瘤或瘤颈开口较宽的囊状动脉瘤,其近端输入动脉可能靠近脾动脉开口或重要分支动脉;若使用覆膜支架,支架近端锚定部分可能覆盖重要分支动脉;若保留分支动脉可能因近端支架锚定距离不够引起内漏,无法达到腔内隔绝的效果;若行栓塞术,弹簧圈或栓塞剂因面对输入动脉高速血流直接冲击无法在瘤体内稳定停留,很大可能脱落至动脉远端导致异位栓塞;此时可采取裸支架+栓塞方法治疗此类SAA。用裸支架覆盖动脉瘤及输入、输出动脉。裸支架上网孔允许血流通过,即使覆盖分支动脉,也可保证其血流通畅。置入裸支架后,用预先留置于瘤体内的导管导入弹簧圈或栓塞剂。此时裸支架起到阻隔作用,将栓塞材料完全拦截在瘤体内而不会移位[24]。另外,因为覆膜支架输送系统直径较粗,当遇到脾动脉直径细小、形态扭曲、或瘤体位于脾动脉远端这类情况时,覆膜支架难以输送,且易损伤动脉。而单纯栓塞对于瘤体较大的SAA无法达到致密填塞的效果。裸支架输送系统尺寸较小,可以通过复杂的脾动脉,配合栓塞技术,即可防止栓塞材料移位,又可保证载瘤动脉通常,特别适合这一类解剖结构复杂的SAA。
以上3种手术方式均有各自的优势与缺点。(1)经导管弹簧圈栓塞术的优势在于:拥有多种多样的尺寸和形状,适用于各种位置和形态的SAA。弹簧圈的缺点在于:栓塞过程中可能伴有部分供血动脉的牺牲和靶器官的梗死[27];本研究的随访结果显示所有术后出现脾梗死的患者均接受过弹簧圈栓塞手术,但此类并发症不会伴有后遗症产生,也不会影响患者生活质量[18]。(2)覆膜支架隔绝术的优势在于:在隔离瘤体的同时,支架内仍可以允许血流通过,从而保证脾动脉主干的通畅和脾脏的灌注,大大降低了脾梗死的风险,符合生理解剖[28]。覆膜支架的缺点在于:因为支架外径较粗,不能进入到脾动脉的远端和分支动脉,且难以通过行程明显扭曲的动脉[28];另外覆膜支架置入术后需长期口服抗血小板药物治疗,伴有潜在出血风险[17]。(3)裸支架+栓塞技术在一定程度上兼顾了弹簧圈和支架隔绝两者的优势;当单纯的栓塞术或支架置入术都有明显缺陷时,可考虑使用裸支架配合弹簧圈的方法进行治疗。目前覆膜支架腔内隔绝术由于其可保留脾动脉的优势在临床上的应用呈逐渐上升的趋势;随着覆膜材料的进步与发展,柔顺性更好、直径及输送系统更细的支架可以很好地突破目前隔绝术所面临的局限。
栓塞综合征是指动脉瘤介入栓塞治疗后,因器官组织局部缺血、代谢或坏死物质吸收,引起患者一系列不适症状及化验结果异常的表现。SAA介入治疗后的栓塞综合征主要有:发热、腹痛、血小板升高、白细胞升高、胸腔积液或急性胰腺炎[29]。腔内治疗术后大约30%的SAA患者会出现栓塞综合征。SAA术后ES不会危及患者生命,但会延长患者住院时间[27]。栓塞综合征的治疗采取对症处理即可,一段时间后这些症状可以逐步缓解并消失,无需再次手术干预。出现栓塞综合征并不意味着患者一定发生了脾梗死;SAA介入术后随访时许多患者发现有脾梗死影像学证据,但患者否认围手术期及出院后有任何自觉症状。近脾门型SAA介入术后发生栓塞综合征和/或脾梗死(有症状或无症状)的风险明显高于其他位置的瘤体[30]。
综上所述,SAA是一种较为少见、发病隐匿、但破裂后病死率较高的动脉疾病。因为SAA在破裂前无症状或症状无特异性,所以诊断需要医师具有相当的临床经验,且诊断有一定的滞后性。对于SAA的治疗方法,腔内治疗在目前的进展较快,已经超过开放手术成为了SAA的首选治疗手段。腔内治疗在局麻下即可进行,手术操作简便,围手术期具有较低的病死率及并发症发生率;手术适应证更广,可用于伴有严重内科疾病、不具备开放手术条件的患者[27];术后患者恢复快,住院时间短;腔内手术住院费用较开放手术稍有增加(5 万/3 万)。根据瘤体的大小、形态、位置,腔内手术可选择覆膜支架腔内隔绝、弹簧圈或栓塞剂栓塞、裸支架+弹簧圈/栓塞剂栓塞等方法。因为SAA为罕见疾病,总体发病率不高,本次研究的病例总数偏少,缺乏足够的病例数对上述3种手术方法进行组间比较,尚不能判断上述3种手术方法中哪一种方法更为安全、有效。另外有相当一部分患者未到我院复查,因此缺乏充足的长期随访的数据。对于SAA各种腔内治疗手段之间优劣的比较仍需要更多的病例数据来进行前瞻性研究。
[1]Uy PPD,Francisco DM,Trivedi A,et al.Vascular Diseases of the Spleen:A Review[J].J Clin Transl Hepatol,2017,5(2):152-164.doi:10.14218/JCTH.2016.00062.
[2]Fang G,Chen B,Fu W,et al.Strategies for endovascular treatment of complicated splenic artery aneurysms[J].J Vasc Surg,2018,68(3):787-794.doi:10.1016/j.jvs.2017.12.053.
[3]Al-Habbal Y,Christophi C,Muralidharan V.Aneurysms of the splenic artery-a review[J].Surgeon,2010,8(4):223-231.doi:10.1016/j.surge.2009.11.011.
[4]Abbas MA,Stone WM,Fowl RJ,et al.Splenic artery aneurysms:two decades experience at Mayo Clinic[J].Ann Vasc Surg,2002,16(4):442-449.doi:10.1007/s10016-001-0207-4.
[5]Hogendoorn W,Lavida A,Hunink MG,et al.Open repair,endovascular repair,and conservative management of true splenic artery aneurysms[J].J Vasc Surg,2014,60(6):1667-1676.doi:10.1016/j.jvs.2014.08.067.
[6]Trastek VF,Pairolero PC,Bernatz PE.Splenic artery aneurysms[J].World J Surg,1985,9(3):378-383.doi:10.1007/BF01655271.
[7]Pulli R,Dorigo W,Troisi N,et al.Surgical treatment of visceral artery aneurysms:a25-year experience[J].J Vasc Surg,2008,48(2):334-242.doi:10.1016/j.jvs.2008.03.043.
[8]Bakhos CT,McIntosh BC,Nukta FA,et al.Staged arterial embolization and surgical resection of a giant splenic artery aneurysm[J].Ann Vasc Surg,2007,21(2):208-210.doi:10.1016/j.avsg.2007.01.005.
[9]Yadav R,Tiwari MK,Mathur RM,et al.Unusually giant splenic artery and vein aneurysm with arteriovenous fistula with hypersplenism in a nulliparous woman[J].Interact Cardiovasc Thorac Surg,2009,8(3):384-386.doi:10.1510/icvts.2008.196121.
[10]Akküçük S,Aydoğan A,Bayarogullari H,et al.Massive upper gastrointestinal bleeding due to giant splenic artery aneurysm with gastric fistula[J].Sakarya Med J,2013,3(3):150-153.doi:10.5505/sakaryamj.2013.62634.
[11]Sadat U,Dar O,Walsh S,et al.Splenic artery aneurysms in pregnancy a systematic review[J].Int J Surg,2008,6(3):261-265.doi:10.1016/j.ijsu.2007.08.002.
[12]Summerour VA,Bramhall SR.Splenic artery aneurysms[J].Adv Emerg Med,2018,8(1):1-2.
[13]Tulsyan N,Kashyap VS,Greenberg RK,et al.The endovascular management of visceral artery aneurysms and pseudoaneurysms[J].J Vasc Surg,2007,45(2):276-283.doi:10.1016/j.jvs.2006.10.049.
[14]Abdulrahman A,Shabkah A,Hassanain M,et al.Ruptured spontaneous splenic artery aneurysm:a case report and review of the literature[J].Int J Surg Case Rep,2014,5(10):754-757.doi:10.1016/j.ijscr.2014.08.021.
[15]Martin D,Farinha HT,Dattner N,et al.Spontaneous non-traumatic splenic artery aneurysm rupture:a case report and review of the literature[J].Eur Rev Med Pharmacol Sci,2018,22(10):3147-3150.doi:10.26355/eurrev_201805_15074.
[16]Stanley JC,Wakefi eld TW,Graham LM,et al.Clinical importance and management of splanchnic artery aneurysms[J].J Vasc Surg,1986,3(5):836-840.
[17]Ouchi T,Kato N,Nakajima K,et al.Splenic Artery Aneurysm Treated With Endovascular Stent Grafting:A Case Report and Review of Literature[J].Vasc Endovascular Surg,2018,52(8):663-668.doi:10.1177/1538574418785252.
[18]Zhu C,Zhao J,Yuan D,et al.Endovascular and Surgical Management of Intact Splenic Artery Aneurysm[J].Ann Vasc Surg,2019,57:75-82.doi:10.1016/j.avsg.2018.08.088.
[19]Morton A.Splenic artery aneurysms,portal hypertension and pregnancy[J].J Hepatol,2019,70(5):1025-1026.doi:10.1016/j.jhep.2018.12.019.
[20]Sendra F,Safran DB,McGee G.A rare complication of splenic artery aneurysm.Mesenteric steal syndrome[J].Arch Surg,1995,130(6):669-672.doi:10.1001/archsurg.1995.01430060107022.
[21]Guillon R,Garcier JM,Abergel A,et al.Management of splenic artery aneurysms and false aneurysms with endovascular treatment in 12 patients[J].Cardiovasc Intervent Radiol,2003,26(3):256-260.doi:10.1007/s00270-003-1948-y.
[22]Berek P,Štefanič P.Surgical Treatment of Splenic Artery Aneurysm[J].Eur J Vasc Endovasc Surg,2017,54(1):11.doi:10.1016/j.ejvs.2017.02.019.
[23]Jesinger RA,Thoreson AA,Lamba R.Abdominal and pelvic aneurysms and pseudoaneurysms:imaging review with clinical,radiologic,and treatment correlation[J].Radiographics,2013,33(3):E71-96.doi:10.1148/rg.333115036.
[24]Hosn MA,Xu J,Sharafuddin M,et al.Visceral Artery Aneurysms:Decision Making and Treatment Options in the New Era of Minimally Invasive and Endovascular Surgery[J].Int J Angiol,2019,28(1):11-16.doi:10.1055/s-0038-1676958.
[25]Jia X,Liu XP,Guo W,et al.The endovascular management of splenic artery aneurysms and pseudoaneurysms[J].Vascular,2011,19(5):257-261.doi:10.1258/vasc.2011.oa0289.
[26]Belli AM,Markose G,Morgan R.The role of interventional radiology in the management of abdominal visceral aneurysms[J].Cardiovasc Interv Radiol,2012,35(2):234-243.doi:10.1007/s00270-011-0201-3.
[27]Venturini M,Marra P,Colarieti A,et al.Covered stenting and transcatheter embolization of splenic artery aneurysms in diabetic patients:A review of endovascular treatment of visceral artery aneurysms in the current era[J].Pharmacol Res,2018,135:127-135.doi:10.1016/j.phrs.2018.07.023.
[28]Venturini M,Marra P,Colombo M,et al.Endovascular Repair of 40 Visceral Artery Aneurysms and Pseudoaneurysms with the Viabahn Stent-Graft:Technical Aspects,Clinical Outcome and Mid-Term Patency[J].Cardiovasc Intervent Radiol,2018,41(3):385-397.doi:10.1007/s00270-017-1844-5.
[29]Piffaretti G,Tozzi M,Lomazzi C,et al.Splenic artery aneurysms:postembolization syndrome and surgical complications[J].Am J Surg,2007,193(2):166-170.doi:10.1016/j.amjsurg.2006.09.007.
[30]Lakin RO,Bena JF,Sarac TP,et al.The contemporary management of splenic artery aneurysms[J].J Vasc Surg,2011,53(4):958-964.doi:10.1016/j.jvs.2010.10.055.
