随着外科技术的提高以及围术期管理的日趋完善,胰十二指肠切除术(pancreaticoduodenectomy,PD)的病死率已降至5%以下,但术后胰瘘(postoperative pancreatic fistula,POPF)的发生率仍高达20%~40%[1-4],严重影响患者的术后恢复,甚至影响远期生存率。研究[5]表明,质软胰腺是发生POPF的重要的危险因素之一。然而,胰腺质地的判断往往凭借医生的主观感受,缺乏客观的评价依据。胰腺星状细胞(pancreatic stellate cells,PSC)与胰腺的纤维化密切相关[6-7]。PSC的活跃度能否用来预测POPF,目前尚无研究报道。本研究前瞻性收集中南大学湘雅医院2017年 12月—2019年9月间101例PD术患者资料,通过分析胰腺切缘组织中PSC的活跃度,探讨PSC的活跃度在PD术后POPF中的预测价值。
1 资料与方法
1.1 一般资料
前瞻性收集2017年12月—2019年9月间中南大学湘雅医院连续收治的101例行PD术的患者,其中男55例(54.5%),女46例(45.5%);年 龄27 ~81岁,中位年龄56 岁;开腹手术96例(95.0%),腹腔镜手术5例(5.0%);经典PD 95例(94.0%),保留幽门的PD(pyloruspreserving PD,PPPD)6例(6.0%);病理类型包括十二指肠乳头癌36例(35.6%),胰腺癌 28例(27.6%),胆总管下段癌11例(10.8%),胰腺囊性肿瘤7例(7.0%),壶腹癌6例(6.0%),慢性胰腺炎6例(6.0%),十二指肠或胰腺间叶源性肿瘤5例(5.0%),胰腺神经内分泌肿瘤1例(1.0%),胆总管炎性狭窄1例(1.0%)。
1.2 染色方法及PSC 活跃度分级
取PD 手术中胰腺颈部切缘处胰腺组织行α-平滑肌肌动蛋白(α-smooth muscle actin,α-SMA)免疫组化染色。取该处胰腺组织石蜡块予以切片、脱蜡后,将其置于柠檬酸钠缓冲溶液(0.01 mol/L,pH6.0)中高压环境(125 ℃, 131 kPa)约15 min以修复抗原。3%H2O2溶液消除内源性过氧化物酶的活性。一抗(鼠抗人单克隆平滑肌肌动蛋白抗体1A4,稀释浓度1∶50),湿盒持续4 ℃孵育过夜。二抗(羊抗鼠单克隆抗体)湿盒孵育,在4 ℃下放置约20~30 min,二氨基联苯胺和苏木精分别进行显色和复染,完成染色。
参考Tanaka等[8]的分级方法,由胰腺病理专家盲法阅片,将α-SMA 免疫组化染色强度分为阴性(-)、弱阳性(+)、中阳性(++)和强阳性(+++)4个等级,分别对应0级(S0)、1级(S1)、2级(S2)、3级(S3)对PSC活跃度进行量化,其具体评判细则如表1所示。
表1 PSC 活跃度的分级标准
Table 1 The grading criteria for PSC activity

PSC 活跃等级 评判细则S0 除胰管周围组织外,无胰腺组织染色,胰腺结构正常S1 一般阳性染色,分布不规则,呈小块斑片状,无明显胰腺结构改变S2 中度阳性染色,分布不均匀,主要位于胰腺小叶内及小叶间,伴腺泡细胞轻度萎缩S3 强阳性染色,分布均匀而弥漫,伴明显腺泡细胞萎缩、小叶结构广泛破坏
本研究中所有的临床数据、标本的收集均获得患者的知情同意,并签署书面同意书。本研究获得中南大学湘雅医院医学伦理委员会批准进行(项目编号:201707777)。
1.3 围术期管理
术后常规预防性使用抗生素以及营养支持等治疗。PD患者术后的腹腔引流管均采用延迟性拔管策略(留置时间≥7 d),并常规于术后第1、3、7天动态监测腹腔引流液淀粉酶(drainage fluid amylase,DFA)情况。对于术后7 d或以后的DFA处于正常范围,且引流管引流量<10 m L/d 的患者,考虑拔除腹腔引流管。所有患者均采取快速康复外科模式促进患者早期恢复[9]。
1.4 POPF 的定义及诊断标准
临床相关性胰瘘(clinically relevant POPF,C R-POPF)的诊断标准参照2016年版胰腺外科国际研究小组[10](International Study Group of Pancreatic Surgery,ISGPS)的定义:术后>3 d时,DFA大于血清淀粉酶正常值上限的3倍,且与临床治疗及预后相关。在此前提下,仅包括B级和C级CR-POPF,而生化漏(biochemical leakage)不包括在内。生化漏:仅术后第3天或以后腹腔引流液淀粉酶升高达正常值上限3倍,而对临床结局无任何影响。B级CR-POPF:(1) 腹腔引流管留置时间>3周;(2) POPF相关性临床治疗方案变更(加用生长抑素、抗生素升级、肠内外营养支持、输血等);(3) 需经皮或内镜下穿刺引流的POPF继发性胰周积液;(4) 需血管造影介入止血的POPF相关性出血;(5) POPF继发性感染,但无脏器功能衰竭。满足以上5种情况中的任意1种。C级CRPOPF:(1) 需再次开放手术处理的POPF相关并发症(如假性动脉瘤破裂出血、腹腔脓肿形成、吻合口破裂等);(2) POPF继发性器官功能衰竭;(3) POPF相关性死亡。满足以上3种情况中的任意 1种。
1.5 统计学处理
计数资料以例数(百分比)[n(%)]表示,其比较采用χ2检验;计量资料中符合正态分布的数据以均数±标准差( ±s)表示,其比较采用 t 检验;符合偏态分布的数据以中位数(M)表示,其比较采用Mann-Whitney U检验。多因素分析采用Logistic回归模型,并应用受试者操作特性曲线(receiver operating characteristic curve,ROC)进行分析。以上均通过SPSS 22.0统计软件分析,P<0.05为差异有统计学意义。
±s)表示,其比较采用 t 检验;符合偏态分布的数据以中位数(M)表示,其比较采用Mann-Whitney U检验。多因素分析采用Logistic回归模型,并应用受试者操作特性曲线(receiver operating characteristic curve,ROC)进行分析。以上均通过SPSS 22.0统计软件分析,P<0.05为差异有统计学意义。
2 结 果
2.1 手术结果
全组101例患者中,41例术后出现CR-POPF,CR-POPF发生率为40.6%,包括B级36例,C 级 5例。无CR-POPF者共60例,包括19例生化漏和41例无胰瘘者。中位手术时长与术中出血量分别为340 min和400 mL。术后总的并发症率为46.5%(47/101),除C R-POPF 外,包括术后胆汁漏 7例(7.0%),延迟性胃排空障碍4例(4.0%),术后出血(包括消化道出血和腹腔内出血)16例(15.8%)和肺部并发症(包括胸腔积液、肺部感染、肺不张及呼吸功能衰竭等)46例(45.5%)。术后中位住院时长为15 d,再入院率为6.0%(6/101),术后90 d内病死率为4.0%(4/101)。
2.2 PSC 活跃度分级
本研究中,切缘处胰腺免疫组化染色的镜下表现及分级如图1 所示。依照评判规则,全组被分级为S0、S1、S2、S3的病例分别为9、49、24、19例,各活跃度等级中的CR-POPF率分别为88.9%(8/9)、57.1%(28/49)、16.7%(4/24)和5.3%(1/19) ,呈逐渐递减趋势,差异有统计学意义(P<0.001)。PSC等级与胰腺质地之间的关系如表2所示。在不同的胰腺质地之间,PSC等级的分布具有明显差异性(P<0.001)。其中,在柔软胰腺中占比最高的PSC等级为S1(60.6%),而在质硬胰腺中为S2(40.0%)。经Spearman相关性检验结果显示,PSC活跃度与胰腺质地的硬度之间存在明显正相关性(r=0.456,P<0.001),而与CR-POPF之间则存在明显负相关性(r=-0.539,P<0.001)。

图1 α-SMA 免疫组化染色所反映的PSC 的活跃度 A:S0;B:S1;C:S2;D:S3
Figure 1 The PSC activity shown by immunohistochemical staining of α-SMA A: S0; B: S1; C: S2; D: S3
表2 PSC 等级与胰腺质地之间的关系[n(%)]
Table 2 Relationship between the PSC activity grade and pancreatic texture [n (%)]
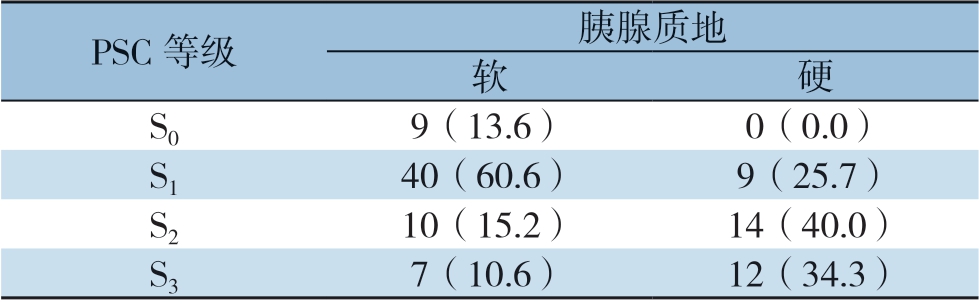
PSC 等级 胰腺质地软硬S0 9(13.6) 0(0.0)S1 40(60.6) 9(25.7)S2 10(15.2) 14(40.0)S3 7(10.6) 12(34.3)
2.3 临床病理因素与CR-POPF 关系的单因素和多元回归分析
单因素分析显示,胰腺质地、肿块病理、PSC活跃度分级、体质量指数(BMI)、胰管直径、术前总胆红素、术后第1 天引流液中淀粉酶含量(DFA1)均与CR-POPF的发生有关(均P<0.05)(表3)。多元回归分析显示,仅PSC活跃度分级(OR=0.24,95% CI=0.10~0.56,P<0.001)和术前总胆红素水平(OR=1.01,95% CI=1.00~1.01,P=0.008)是预测CR-POPF的独立危险因素(表4)。
2.4 PSC 活跃度分级对CR-POPF 的预测价值
PSC活跃度分级预测CR-POPF的ROC曲线如图2 所示。当截断值取1.5 时,其曲线下面积(area under curve,AUC)为0.795(95% CI=0.708~0.881),相应的敏感度和特异度分别为63.3%和87.8%,提示其对CR-POPF具有良好的预测价值。
表3 临床病理因素与CR-POPF 关系的单因素分析
Table 3 Univariate analysis of relationship between clinicopathologic factors and CR-POPF
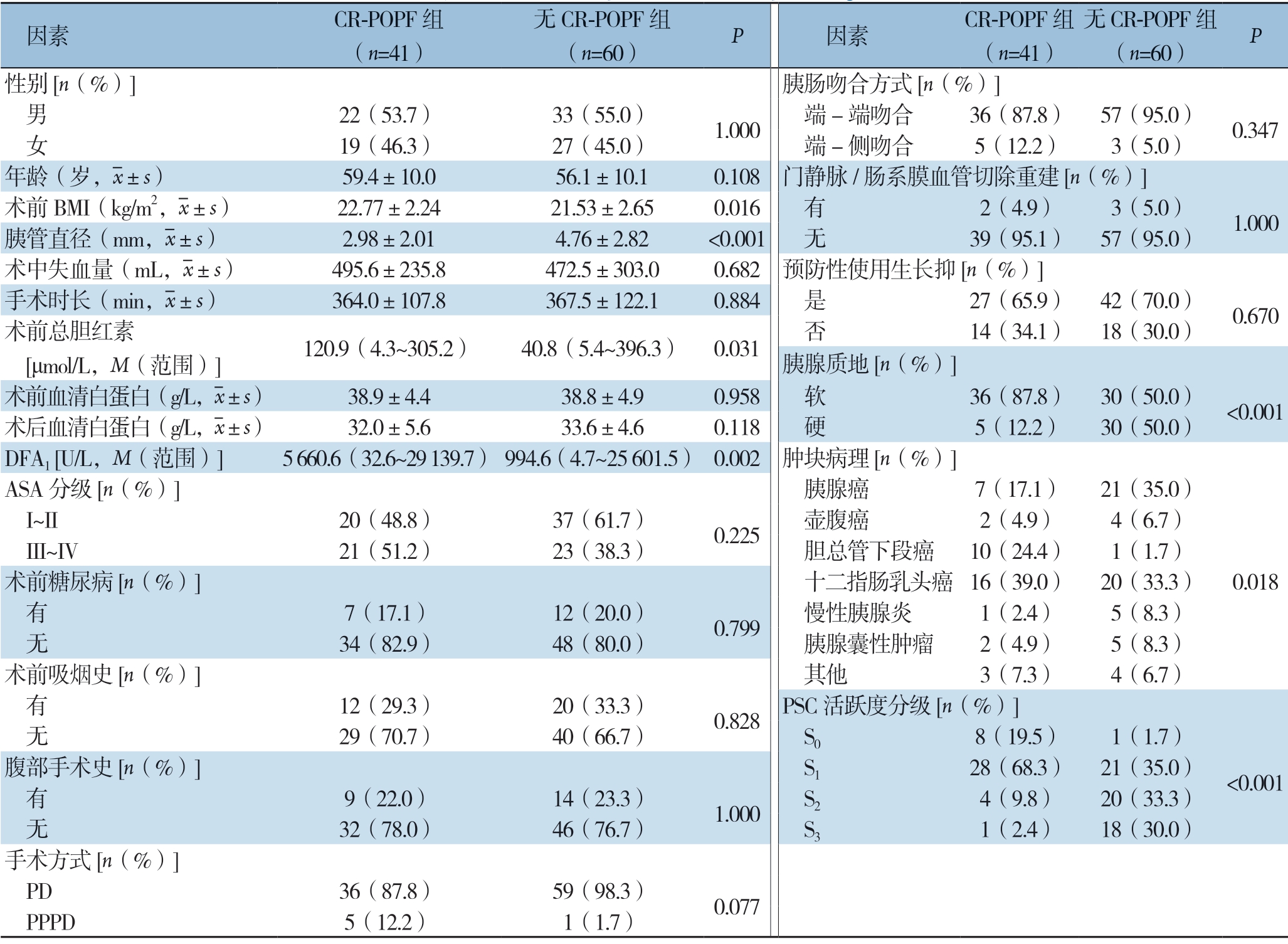
因素 CR-POPF 组(n=41)无CR-POPF 组(n=60) P 因素 CR-POPF 组(n=41)无CR-POPF 组(n=60) P性别[n(%)] 胰肠吻合方式[n(%)] 男 22(53.7) 33(55.0) 1.000 端-端吻合 36(87.8) 57(95.0) 0.347 女 19(46.3) 27(45.0) 端-侧吻合 5(12.2) 3(5.0)年龄(岁,images/BZ_33_1708_490_1742_536.png±s) 59.4±10.0 56.1±10.1 0.108 门静脉/肠系膜血管切除重建[n(%)]术前BMI(kg/m2,images/BZ_33_1708_490_1742_536.png±s) 22.77±2.24 21.53±2.65 0.016 有 2(4.9) 3(5.0) 1.000胰管直径(mm,images/BZ_33_1708_490_1742_536.png±s) 2.98±2.01 4.76±2.82 <0.001 无 39(95.1) 57(95.0)术中失血量(mL,images/BZ_33_1708_490_1742_536.png±s) 495.6±235.8 472.5±303.0 0.682 预防性使用生长抑[n(%)]手术时长(min,images/BZ_33_1708_490_1742_536.png±s) 364.0±107.8 367.5±122.1 0.884 是 27(65.9) 42(70.0) 0.670术前总胆红素 [μmol/L,M(范围)] 120.9(4.3~305.2) 40.8(5.4~396.3) 0.031 否 14(34.1) 18(30.0)胰腺质地[n(%)]术前血清白蛋白(g/L,images/BZ_33_1708_490_1742_536.png±s) 38.9±4.4 38.8±4.9 0.958 软 36(87.8) 30(50.0) <0.001术后血清白蛋白(g/L,images/BZ_33_1708_490_1742_536.png±s) 32.0±5.6 33.6±4.6 0.118 硬 5(12.2) 30(50.0)DFA1 [U/L,M(范围)] 5660.6(32.6~29139.7) 994.6(4.7~25601.5) 0.002 肿块病理[n(%)]ASA 分级[n(%)] 胰腺癌 7(17.1) 21(35.0)0.018 I~II 20(48.8) 37(61.7) 0.225 壶腹癌 2(4.9) 4(6.7) III~IV 21(51.2) 23(38.3) 胆总管下段癌 10(24.4) 1(1.7)术前糖尿病[n(%)] 十二指肠乳头癌 16(39.0) 20(33.3) 有 7(17.1) 12(20.0) 0.799 慢性胰腺炎 1(2.4) 5(8.3) 无 34(82.9) 48(80.0) 胰腺囊性肿瘤 2(4.9) 5(8.3)术前吸烟史[n(%)] 其他 3(7.3) 4(6.7) 有 12(29.3) 20(33.3) 0.828 PSC 活跃度分级[n(%)] 无 29(70.7) 40(66.7) S0 8(19.5) 1(1.7)腹部手术史[n(%)] S1 28(68.3) 21(35.0) <0.001 有 9(22.0) 14(23.3) 1.000 S2 4(9.8) 20(33.3) 无 32(78.0) 46(76.7) S3 1(2.4) 18(30.0)手术方式[n(%)] PD 36(87.8) 59(98.3) 0.077 PPPD 5(12.2) 1(1.7)
表4 临床病理因素与CR-POPF 关系多因素Logistic 回归分析
Table 4 Multivariate Logistic regression analysis of relationship between clinicopathologic factors and CR-POPF

因素 OR 95% CI PBMI 1.15 0.91~1.45 0.224胰腺质地(软) 2.39 0.55~9.28 0.245肿块病理 1.88 0.44~8.08 0.357胰管直径 0.98 0.75~1.28 0.428术前总胆红素 1.01 1.00~1.01 0.008 DFA1 1.00 1.00~1.00 0.441 PSC 活跃度分级 0.24 0.10~0.56 <0.001
3 讨 论

图2 PSC 等级预测PD 术后CR-POPF 的ROC 曲线
Figure 2 ROC curve of PSC activity for predicting CR-POPF following PD
POPF是PD术后的严重并发症之一,可继发引起术后出血、腹腔感染、延迟性胃排空障碍等一系列并发症,甚至导致患者死亡[11-15]。胰腺质地与POPF的发生密切相关[5,16]。然而,仅靠外科医生的触感判断胰腺质地较为主观。因此,国内外均有研究采用其它较为客观的方法来评判胰腺质地,如MRI、胰腺弹力仪等,取得一定效果,但实用性不高[17-21]。PSC作为胰腺实质中的基质细胞,活化后可分泌大量纤维组织,从而参与胰腺组织的纤维化,理论上可能与胰腺质地变硬密切相关[6-7,21-22]。本研究采用免疫组化检测胰腺切缘组织中的α-SMA蛋白反映PSC的活跃度,前瞻性研究101例PD患者的临床资料,发现PSC活跃度是预测CR-POPF的独立危险因素(OR=0.24,95% CI=0.10~0.56,P<0.001)。此外,ROC曲线分析显示PSC活跃度的预测效能良好(AUC=0.795),从而进一步证实 PSC活跃度是预测PD术后胰瘘的重要的客观指标。
PSC是一种外形类似星形的胰腺基质细胞,最初于1998年被发现并分离培养[23-24]。它存在于胰腺腺泡细胞、微血管及微胰管周围。在大多数情况下,PSC处于静止态,具有免疫调节、吞噬、储存脂质、维持胰腺组织基础内外分泌以及其基本结构等功能[25-26]。然而,在缺氧、酒精、吸烟等恶劣条件刺激下,PSC可转为活化态并获得成纤维细胞样表型[27-30],分泌大量细胞因子(如TNF-α,TGF-β、PDGF等)以及细胞外基质,促进胰腺组织的纤维化进程[7,31]。较为特殊的是,慢性胰腺炎的胰腺组织中有大量单核细胞浸润,后者通过产生TNF-α促进PSC活化[32];胰腺癌细胞与PSC存在纤维化/缺氧循环机制[33],通过正反馈放大作用致使组织持续性的缺氧、缺血、纤维化。以上可能是PSC介导胰腺纤维化的主要机制。然而,其更具体的分子信号通路仍有待于进一步探究。
从组织病理学角度来看,胰腺质地取决于组织中的纤维化程度,它与胰腺的外分泌功能呈负相关性[22,34]。然而究其根本,胰腺纤维组织的蓄积是PSC活跃的结果,故后者更能反映胰腺质地变化的本质。遗憾的是,目前的研究主要集中于探索胰腺纤维化与胰瘘的联系[35-37],而鲜有关于PSC活跃度和POPF关联性的研究。Erkan等[38]认为胰腺癌中的PSC是导致胰腺纤维化的主要原因,可影响外科医生对质地的判断。Tanaka等[8]通过将不同活跃程度的PSC进行分级并对CR-POPF进行预测,率先证实PSC等级在发生POPF与无POPF的患者之间存在统计学差异(P=0.035)。在此基础上,本研究发现随着PSC活跃度提高,CR-POPF率明显降低,这与前述的理论基本契合。而且,在本研究中,PSC活跃度被证实是发生CR-POPF的独立危险因素,而胰腺质地并不是独立的危险因素,从而证实了PSC活跃度在预测CR-POPF方面更具有客观性与优越性。因此,PSC活跃度可代替胰腺质地,作为评估CR-POPF风险的全新预测指标。
本研究存在一定的局限性。首先,本研究所涵盖的样本量不大,且为单中心研究;其次,PSC活跃时分泌或活化的蛋白种类繁多,其特征性蛋白除α-SMA外[39],还包括成纤维细胞活化蛋白-α,潜在转化生长因子结合蛋白等[40-41]。本研究中的标记蛋白较为单一,其客观度稍显不足,有待于在今后的实验中予以完善。
综上所述,PSC的活跃度对CR-POPF的预测具有重要意义,可作为临床指导预测CR-POPF发生的重要参考指标之一。
[1] Kawakatsu S, Inoue Y, Mise Y, et al.Comparison of pancreatojejunostomy techniques in patients with a soft pancreas: Kakita anastomosis and Blumgart anastomosis[J].BMC Surg, 2018, 18(1):88.doi: 10.1186/s12893-018-0420-5.
[2] Ecker BL, McMillan MT, Asbun HJ, et al.Characterization and optimal management of high-risk pancreatic anastomoses during pancreatoduodenectomy[J].Ann Surg, 2018, 267(4):608-616.doi: 10.1097/SLA.0000000000002327.
[3] Allen PJ, Gönen M, Brennan MF, et al.Pasireotide for postoperative pancreatic fistula[J].N Engl J Med, 2014, 370(21):2014-2022.doi: 10.1056/NEJMoa1313688.
[4] Ellis RJ, Brock Hewitt D, Liu JB, et al.Preoperative risk evaluation for pancreatic fistula after pancreaticoduodenectomy[J].J Surg Oncol, 2019, 119(8): 1128-1134.doi: 10.1002/jso.25464.
[5] Hamanaka Y, Nishihara K, Hamasaki T, et al.Pancreatic juice output after pancreatoduodenectomy in relation to pancreatic consistency, duct size, and leakage[J].Surgery, 1996, 119(3):281-287.doi: 10.1016/s0039-6060(96)80114-0.
[6] Masamune A, Watanabe T, Kikuta K, et al.Roles of pancreatic stellate cells in pancreatiCInflammation and fibrosis[J].Clin Gastroenterol Hepatol, 2009, 7(11):S48-54.doi: 10.1016/j.cgh.2009.07.038.
[7] Bachem MG, Schünemann M, Ramadani M, et al.Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells[J].Gastroenterology, 2005, 128(4):907-921.doi: 10.1053/j.gastro.2004.12.036.
[8] Tanaka K, Tomita H, Osada S, et al.Significance of histopathological evaluation of pancreatic fibrosis to predict postoperative course after pancreatic surgery[J].Anticancer Res, 2015, 35(3):1749-1756.
[9] Lassen K, Coolsen MM, Slim K, et al.Guidelines for perioperative care for pancreaticoduodenectomy: enhanced recovery after surgery (ERAS) society recommendations[J].Clin Nutr, 2012, 31(6):817-830.doi: 10.1016/j.clnu.2012.08.011.
[10] Bassi C, Marchegiani G, Dervenis C, et al.The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After[J].Surgery, 2017, 161(3):584-591.doi: 10.1016/j.surg.2016.11.014.
[11] El Nakeeb A, El Sorogy M, Hamed H, et al.Biliary leakage following pancreaticoduodenectomy: prevalence, risk factors and management[J].Hepatobiliary Pancreat Dis Int, 2019, 18(1):67-72.doi: 10.1016/j.hbpd.2018.10.005.
[12] 郑岩松, 石铮, 翁山耕, 等.胰十二指肠切除术后腹腔内迟发性出血的原因与处理[J].中国普通外科杂志, 2011, 20(6):626-628.
Zheng YS, Shi Z, Weng SG, et al.The causes and treatment of delayed intra-abdominal hemorrhage after pancreatoduodenectomy[J].Chinese Journal of General Surgery, 2011, 20(6):626-628.
[13] 刘国华, 谭小宇, 戴东, 等.改良Blumgart胰肠吻合在胰十二指肠切除术中的应用[J].中国普通外科杂志, 2020, 29(3):276-283.doi:10.7659/j.issn.1005-6947.2020.03.004.
Liu GH, Tan XY, Dai D, et al.Application of modified Blumgart pancreaticojejunostomy in pancreaticoduodenectomy[J].Chinese Journal of General Surgery, 2020, 29(3):276-283.doi:10.7659/j.issn.1005-6947.2020.03.004.
[14] Nagakawa Y, Matsudo T, Hijikata Y, et al.Bacterial contamination in ascitic fluid is associated with the development of clinically relevant pancreatic fistula after pancreatoduodenectomy[J].Pancreas, 2013, 42(4):701-706.doi: 10.1097/MPA.0b013e31826d3a41.
[15] Williamsson C, Ansari D, Andersson R, et al.Postoperative pancreatic fistula-impact on outcome, hospital cost and effects of centralization[J].HPB (Oxford), 2017, 19(5):436-442.doi: 10.1016/j.hpb.2017.01.004.
[16] Sugimoto M, Takahashi S, Kojima M, et al.In patients with a soft pancreas, a thick parenchyma, a small duct, and fatty infiltration are significant risks for pancreatic fistula after pancreaticoduodenectomy[J].J Gastrointest Surg, 2017, 21(5):846-854.doi: 10.1007/s11605-017-3356-7.
[17] Maehira H, Iida H, Mori H, et al.Computed tomography enhancement pattern of the pancreatic parenchymAPredicts postoperative pancreatic fistula after pancreaticoduodenectomy[J].Pancreas, 2019, 48(2):209-215.doi: 10.1097/MPA.0000000000001229.
[18] Watanabe H, Kanematsu M, Tanaka K, et al.Fibrosis and postoperative fistula of the pancreas: correlation with MR imaging findings--preliminary results[J].Radiology, 2014, 270(3):791-799.doi: 10.1148/radiol.13131194.
[19] Hong TH, Choi JI, Park MY, et al.Pancreatic hardness: correlation of surgeon's palpation, durometer measurement and preoperative magnetic resonance imaging features[J].World J Gastroenterol, 2017, 23(11):2044-2051.doi: 10.3748/wjg.v23.i11.2044.
[20] Shi Y, Liu Y, Gao F, et al.Pancreatic stiffness quantified with MR elastography: relationship to postoperative pancreatic fistula after pancreaticoenteric anastomosis[J].Radiology, 2018, 288(2):476-484.doi: 10.1148/radiol.2018170450.
[21] Marchegiani G, Ballarin R, Malleo G, et al.Quantitative assessment of pancreatic texture using a durometer: A new tool to predict the risk of developing APostoperative fistula[J].World J Surg, 2017, 41(11):2876-2883.doi: 10.1007/s00268-017-4073-9.
[22] Pereira FL, Vasques FT, Moricz Ad, et al.Correlation analysis between post-pancreatoduodenectomy pancreatic fistula and pancreatic histology[J].Rev Col Bras Cir, 2012, 39(1):41-47.
[23] Apte MV, Haber PS, Applegate TL, et al.Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture[J].Gut, 1998, 43(1):128-133.doi: 10.1136/gut.43.1.128.
[24] Bachem MG, Schneider E, Gross H, et al.Identification, culture, and characterization of pancreatic stellate cells in rats and humans[J].Gastroenterology, 1998, 115(2):421-432.doi: 10.1016/s0016-5085(98)70209-4.
[25] Nielsen MFB, Mortensen MB, Detlefsen S, et al.Identification of markers for quiescent pancreatic stellate cells in the normal human pancreas[J].Histochem Cell Biol, 2017, 148(4):359-380.doi: 10.1007/s00418-017-1581-5.
[26] Ferdek PE, Jakubowska MA.Biology of pancreatic stellate cells-more than just pancreatic cancer[J].Pflugers Arch, 2017, 469(9):1039-1050.doi: 10.1007/s00424-017-1968-0.
[27] Rebours V, Albuquerque M, Sauvanet A, et al.HypoxiAPathways and cellular stress activate pancreatic stellate cells: development of an organotypic culture model of thick slices of normal human pancreas[J].PLoS One, 2013, 8(9): e76229.doi: 10.1371/journal.pone.0076229.
[28] Haber PS, Keogh GW, Apte MV, et al.Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis[J].Am J Pathol, 1999, 155(4):1087-1095.doi: 10.1016/S0002-9440(10)65211-X.
[29] Hashimoto A, Karim MR, Izawa T, Kuwamura M, Yamate J.Immunophenotypical analysis of pancreatiCInterstitial cells in the developing rat pancreas and myofibroblasts in the fibrotic pancreas in dogs and cats[J].J Vet Med Sci, 2017, 79(12):1920-1926.doi: 10.1292/jvms.17-0423.
[30] Öhlund D, Handly-Santana A, Biffi G, et al.Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer[J].J Exp Med, 2017, 214(3):579-596.doi: 10.1084/jem.20162024.
[31] Laklai H, Miroshnikova YA, Pickup MW, et al.Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression[J].Nat Med, 2016, 22(5):497-505.doi: 10.1038/nm.4082.
[32] Michalski CW, Gorbachevski A, Erkan M, et al.Mononuclear cells modulate the activity of pancreatic stellate cells which in turn promote fibrosis and inflammation in chronic pancreatitis[J].J Transl Med, 2007, 5:63.doi: 10.1186/1479-5876-5-63.
[33] Erkan M, Reiser-Erkan C, Michalski CW, et al.Cancer-stellate cell interactions perpetuate the hypoxia-fibrosis cycle in pancreatic ductal adenocarcinoma[J].Neoplasia, 2009, 11(5):497-508.doi: 10.1593/neo.81618.
[34] Deng Y, Zhao B, Yang M, et al.Association between the incidence of pancreatic fistula after pancreaticoduodenectomy and the degree of pancreatic fibrosis[J].J Gastrointest Surg, 2018, 22(3):438-443.doi: 10.1007/s11605-017-3660-2.
[35] Umezaki N, Hashimoto D, Nakagawa S, et al.Number of acinar cells at the pancreatic stump predicts pancreatic fistula after pancreaticoduodenectomy[J].Surg Today, 2018, 48(8):790-795.doi: 10.1007/s00595-018-1656-5.
[36] Nahm CB, Lui I, Naidoo CS, et al.Density and enhancement of the pancreatic tail on computer tomography predicts acinar score and pancreaticfistula after pancreatoduodenectomy[J].HPB (Oxford), 2019, 21(5):604-611.doi: 10.1016/j.hpb.2018.09.014.
[37] Kang JH, Park JS, Yu JS, et al.Prediction of pancreatic fistula after pancreatoduodenectomy by preoperative dynamic CT and fecal elastase-1 levels[J].PLoS One, 2017, 12(5):e0177052.doi: 10.1371/journal.pone.0177052.
[38] Erkan M, Hausmann S, Michalski CW, et al.How fibrosis influences imaging and surgical decisions in pancreatic cancer[J].Front Physiol, 2012, 3:389.doi: 10.3389/fphys.2012.00389.
[39] Fujita H, Ohuchida K, Mizumoto K, et al.Alpha-smooth muscle actin expressing stromAPromotes an aggressive tumor biology in pancreatic ductal adenocarcinoma[J].Pancreas, 2010, 39(8):1254-1262.doi: 10.1097/MPA.0b013e3181dbf647.
[40] Fu Y, Liu S, Zeng S, et al.The critical roles of activated stellate cells-mediated paracrine signaling, metabolism and oncoimmunology in pancreatic ductal adenocarcinoma[J].Mol Cancer, 2018, 17(1):62.doi: 10.1186/s12943-018-0815-z.
[41] Sarper M, Cortes E, Lieberthal TJ, et al.ATRA modulates mechanical activation of TGF-β by pancreatic stellate cells[J].Sci Rep, 2016, 6:27639.doi: 10.1038/srep27639.
