以新生内膜高度增生为特征的血管负性重塑,是动脉缺血性疾病血运重建后再狭窄的主要原因,抑制新生内膜高度增生的方法如血小板生长因子受体拮抗剂、药物洗脱支架、分子靶向治疗(如E2F转录因子诱导剂)等在临床上的应用效果并不如预期所料[1-3],因此亟需另辟蹊径,探寻新的调节动脉损伤后负性重塑过程的机制,为解决动脉损伤后再狭窄问题提供参考方向。
肠道菌群在维持人体环境稳态和肠道屏障功能、调节肠道免疫系统、影响营养吸收和能量代谢等方面发挥着重要作用[4-6],近年研究发现,肠道菌群衍生的代谢产物氧化三甲胺(trimethylamine oxide,TMAO)水平与心血管疾病风险存在关联,TMAO血浆浓度升高具有预测未来不良心血管疾病结局的价值[7-8]。TMAO水平升高会促进血管炎症反应,诱发动脉粥样硬化、加剧压力负荷引起心力衰竭、或中风甚至延长血管紧张素II的作用引起高血压[7,9-12],但暂未涉及TMAO水平与动脉损伤后重塑过程的调控关系。本研究通过建立动脉损伤模型,探讨肠道菌群代谢物TMAO在动脉内膜特异性增生过程中的作用机制,验证TMAO加速动脉内膜损伤后负性重塑的假设。
1 材料与方法
1.1 材料
成年野生型SD大鼠50只,雌雄随机,体质量350~400 g,由昆明医科大学SPF实验中心提供,大鼠均在“昆明医科大学实验动物饲养与操作规程”下规范实验操作。TSQ Quantum 三重四极杆质谱仪,UltiMate 3000 RS色谱仪,美国赛默飞世尔科技;eNOS(AF0096,1:1000,Affinity),p-e NOS(Ser-1177)(AF 3247,1:1000,Affinity),CD31(AF6191,1:200,Affinity);ROS染液(D7008,1:500,SIGMA)。
1.2 方法
1.2.1 构建大鼠腹主动脉球囊损伤模型 大鼠适应性单笼饲养1 周,以20 g/L 戊巴比妥钠50 mg/kg腹腔麻醉,显露腹主动脉,于肾动脉水平暂时阻断血流,逆行将0.014 mm 导丝自髂动脉分叉处插至膈肌水平,导丝引导下2.5 mm×20 mm 球囊扩张导管插入腹主动脉约2~2.5 cm,压力泵以6 ATM充盈球囊缓慢来回抽动2 次,旋转180°再次缓慢来回抽动2 次剥脱内膜。
1.2.2 分组及处理 将50 只大鼠随机分为对照组、模型组、TMAO 增强组、TMAO 抑制组、粪菌移植组,每组各10 只。模型组仅作腹主动脉球囊损伤;TMAO 增强组术后予1.5%TMAO 水溶液喂养[13];TMAO 抑制组术后予1.0%DMB[ 减少三甲胺(trimethylamine,TMA)形成而降低TMAO 水平]水溶液喂养[14];粪菌移植组按照谈路轩等[15]经验提取粪菌移植液:取正常大鼠成形新鲜粪便50 g,尽快加入200 mL 生理盐水,置于搅拌器中进行搅拌,经2 层无菌纱布去除其中大颗粒物质,将样本置入匀质器中,使用逐层缩小的过滤网去除食物残渣和小颗粒物质,将样本以6000 r/min 离心15 min,去除沉淀,取上清1/2 得最终的粪菌液提取液,-20 ℃保存备用,术后经灌肠途径予菌液移植治疗,每周2 次。所有组每天更换饮用水,直至4 周后取标本。
1.2.3 血浆TMAO 检测 取标本前禁食12 h,不禁水,常规麻醉后穿刺腹主动脉,将血样收集到EDTA抗凝血管中,样品立即3000 r/min 离心15 min,血浆-80 ℃保存,采用液相色谱- 串联质谱仪(LC/MS)测定TMAO 浓度[16]。
1.2.4 组织染色及检测 取血后过量麻醉大鼠致死,迅速取损伤段腹主动脉,并将其置于冰盐水中,清除脂肪和结缔组织,进行组织病理染色。应用NIH Image J 医学图象分析系统,测量损伤段动脉管壁厚度、新生内膜厚度(HE 染色)、弹力纤维鉴定(EVG 染色)、血小板- 内皮细胞黏附分子(CD31)免疫组化染色,以上染色方法均按云南省阜外心血管病医院病理科操作规程进行。Western blot 检测内皮依赖型一氧化氮合酶(endothelial nitric oxide synthase,eNOS)和磷酸化eNOS 表达及免疫荧光测定活性氧(reactive oxygen species,ROS)半定量分析均按试剂盒操作规程完成。
1.3 统计学处理
TMAO浓度的色谱图采集和积分由软件Xcalibur 3.0(Thermo)进行处理,以1/χ2为加权系数进行线性回归。各组实验独立重复3次,数据以均数±标准差(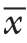 ±s)表示,应用IBM SPSS 23.0统计软件分析,多组间总体比较采用单因素方差分析,组间比较采用LSD-t检验,P<0.05为差异有统计学意义。
±s)表示,应用IBM SPSS 23.0统计软件分析,多组间总体比较采用单因素方差分析,组间比较采用LSD-t检验,P<0.05为差异有统计学意义。
2 结果
2.1 血浆TMAO 水平
建模成功后取大鼠动脉血,LC/MS测定血浆TMAO浓度。与正常对照组比较,模型组、TMAO增强组、TMAO抑制组血浆TMAO浓度均升高,TMAO增强组升高尤为明显,差异均有统计学意义(均P<0.05);粪菌移植组血浆TMAO浓度较正常对照组比较差异无统计学意义(P>0.05)(图1)。

图1 LC/MS 检测血浆TMAO 浓度
Figure 1 Plasma TMAO levels determined by LC/MS
2.2 组织染色
建模成功后取损伤段腹主动脉,HE染色测量内膜(I)+中膜(M)厚度(像素):正常对照组I+M=450.40±66.42,模型组I+M=775.33±341.49,TMAO增强组I+M=923.73±199.05,TMAO抑制组I+M=636.84±192.46,粪菌移植组I+M=682.88±174.37;与正常对照组比较,各组内膜不同程度增厚,可见泡沫细胞,其中以TMAO增强组内膜增生程度最为明显(均P<0.05),部分管腔可见狭窄近闭塞结果。EVG染色见正常对照组主动脉壁结构完整、层次清晰,弹性纤维排列紧密、有序,弹力板结构完整(IOD=131.89±4.40);与正常对照组比较,模型组(IOD=123.02±3.34)、TMAO增强组(IOD=117.06±3.27)、TMAO抑制组(IOD=124.10±6.65)及粪菌移植组(IOD=121.78±3.24)均可见弹力纤维有不同程度破碎、排列紊乱,弹力板层断裂、丢失,其中TMAO增强组弹力纤维崩解程度最为明显(均P<0.05)。CD31是内皮细胞的特异性标志物,对维持血管内皮细胞间的连接及维持血管完整性具有重要作用。CD31免疫组化染色见:TMAO增强组CD31阳性内皮细胞组化评分最高(H-score=28.59±10.16),但各组间差异均无统计学意义(均P>0.05)(图2)。

图2 各组腹主动脉标本染色结果(×200,比例尺:100 μm)
Figure 2 Staining results of sample of the abdominal aorta from each(×200,scale bar:100 μm)
2.3 eNOS 和磷酸化eNOS
NO在改善血管内皮通透性、抑制内膜新生和管壁增厚方面具有重要作用,NO主要由eNOS在内皮细胞内催化L-精氨酸合成,而丝氨酸1177(Ser1177)磷酸化激活eNOS[17]。为了检测腹主动脉球囊损伤模型中循环TMAO升高是否会降低eNOS活性,检测了损伤段动脉组织中的总eNOS和eNOS在丝氨酸第1177 位点磷酸化(p-eNOS Ser1177)的表达。结果发现,各组总eNOS表达差异均无统计学意义(均P>0.05),然而,p-eNOS Ser1177在模型组及TMAO增强组明显减少,尤其以TMAO增强组降低程度最明显(均P<0.05),而与正常对照组比较,TMAO抑制组及粪菌移植组磷p-eNOS Ser1177水平差异均无统计学意义(均P>0.05)(图3)。
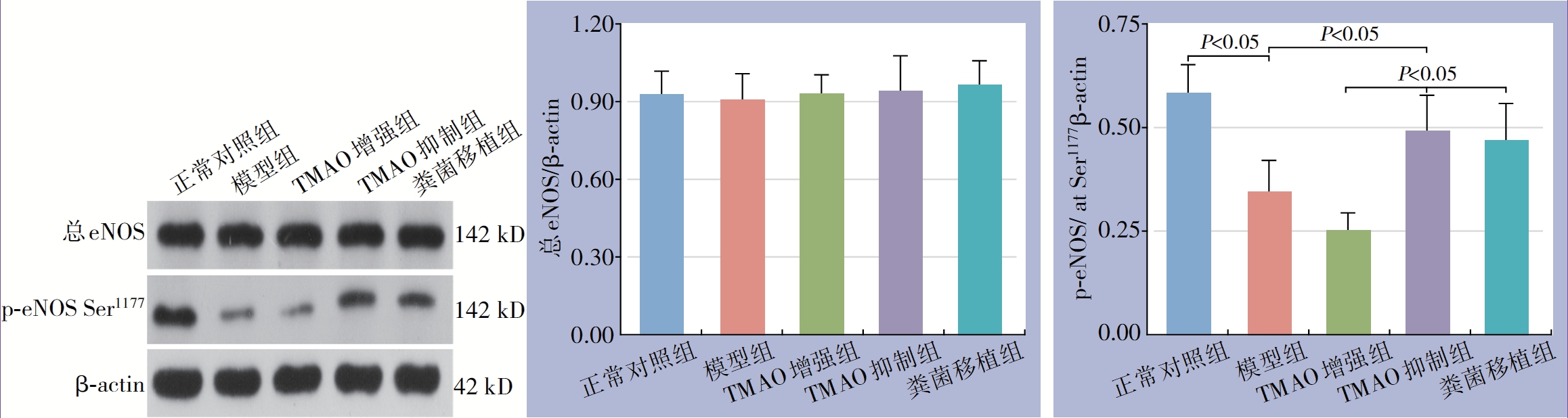
图3 Western blot 检测eNOS 和eNOS Ser1177 的表达
Figure 3 Western blot analysis of expressions of eNOS and p-eNOS Ser1177
2.4 ROS 半定量分析
ROS可以迅速灭活NO,并氧化eNOS辅因子导致eNOS和氧化蛋白的致病解偶,为探究TMAO干预后是否会引起损伤段动脉ROS变化,利用荧光探针DCFH-DA进行ROS检测,结果显示:与正常对照组比较,球囊损伤后动脉内膜层荧光强度高于中膜-外膜层,模型组、TMAO增强组、TMAO抑制组ROS阳性细胞数减低,其中以TMAO组减低程度最大(均P<0.05),而粪菌移植组ROS阳性细胞数较正常对照组差异无统计学意义(P>0.05)(图4)。

图4 免疫荧光测定ROS 阳性细胞数(×200,比例尺:100 μm)
Figure 4 Determination of ROS positive cells by immunofluorescence(×200,scale bar:100 μm)
3 讨论
血管腔内技术飞速发展,动脉缺血性疾病的治疗策略不断更新,但以新生内膜高度增生为特征的血管负性重塑,最终仍导致大多数患者出现术后动脉管腔再狭窄,严重影响手术的远期疗效。因此,了解导致动脉损伤后负性重塑的机制,对于临床制定治疗方案,使更多的血管成形术患者受益具有重要意义。对此诸多学者研究通过干预新生内膜的某一环节以达到控制血管内膜过度增生的目的,H u 等[18]通过调控血管内皮生长因子(vascular endothelial growth factor,VEGF)表达以调控细胞再内皮化;Yang等[19]研发的新型雷帕霉素负载血管移植物、Ye等[20]通过调控lcnRNA KCNQ1OT1表达,以干预血管平滑肌细胞功能来抑制新生内膜形成,但目前还处于动物实验阶段;同样地,邬光敏等[21]研究发现,稳定静脉分子指纹EphB4的持续表达能维持静脉属性,移植静脉增生程度降低;再如血小板生长因子受体拮抗剂、药物洗脱支架、分子靶向治疗等,因其费用高昂等原因致使在临床上转化应用受限,因此亟需探索新的干预方向。
机体摄入的含磷脂酰胆碱的食物,经肠道菌群作用形成三甲胺(trimethylamine,TMA),TMA经门静脉循环进入肝脏,在肝脏黄酮单氧化酶作用下迅速氧化生成TMAO,经肾小球滤过和肾小管分泌被肾脏排出[7]。目前大量研究发现TMAO与心血管不良事件的发生密切相关[22-23],肠道菌群失调、手术应激都可能导致TMAO产量的增加[24],Chou等[25]发现TMAO增加能抑制内皮祖细胞的产生导致内皮细胞功能障碍,Ke等[13]研究证实循环TMAO升高,内皮细胞增殖程度降低,血管功能和重塑能力下降;另外,TMAO亦可引起炎症反应,并加速动脉粥样硬化形成[26-27]。前期大量研究确定了本实验中目标研究因子在动脉损伤后重塑过程作用机制的可行性,但主要集中于内皮细胞生物学行为以及TMAO与血管发育的研究,暂未涉及TMAO与血管损伤后重塑过程的机制研究。本实验发现,模型组大鼠腹主动脉球囊损伤术后,血浆TMAO浓度较正常对照组升高,而予菌液治疗,调节肠道菌群后,血浆TMAO浓度较正常对照组差异无统计学意义。病理染色结果显示,损伤段腹主动脉管腔与正常对照组相比,管腔形态不规则,球囊损伤术后各组均有不同程度新生内膜形成,其中以TMAO增强组I+M=923.73±199.05,增生程度最重、弹力纤维IOD=117.06±3.27,崩解程度最为明显(P<0.001),CD31阳性内皮细胞组化显示球囊损伤术后内皮细胞增生,在TMAO增强组中评分最高(H-score=28.59±10.16),说明TMAO干预后会促进内皮细胞病理性增殖、迁移,促进新生内膜形成,弹力纤维崩解致管腔舒缩能力降低,管腔负性重塑加速。TMAO抑制组I+M=636.84±192.46,粪菌移植组I+M=682.88±174.37,新生内膜增生程度降低,进一步证实调节肠道菌群稳定后TMAO生成减少能减缓受损管腔的负性重塑。
理想情况下,ECs位于血流和血管壁的边界,调节血管主要的稳态特性,包括止血、溶栓、炎症反应、血管活性、血管通透性和血管重构,是血管成形术后创面愈合的关键步骤,因此,稳定ECs功能是处理动脉损伤后的关键点。NO在eNOS作用下形成,经ROS灭活,已证实在改善血管内皮通透性、抑制内膜新生和管壁增厚方面具有重要作用。为进一步探究TMAO引起动脉新生内膜高度增生的作用机制,以NO的生成和灭活途径为研究点,首先,NO主要由eNOS在内皮细胞内催化L-精氨酸合成,而Ser1177磷酸化激活eNOS [17],本研究检测了总eNOS和eNOS Ser1177位点磷酸化表达量,发现各组总eNOS表达无显著差异,而eNOS Ser1177位点磷酸化在模型组及TMAO增强组显著减少,尤其以TMAO增强组降低程度最明显,而与正常对照组相比,TMAO抑制组及粪菌移植组磷酸化水平差异均无统计学意义,说明TMAO作用于eNOS Ser1177位点,通过降低其磷酸化影响eNOS活性。但本研究中eNOS Ser1177位点磷酸化水平在TMAO干预后显著降低,而总eNOS表达量无显著差异,可能的原因是eNOS的活化不仅仅依赖于Ser1177位点磷酸化。既往研究发现,Thr497磷酸化状态可能平衡eNOS刺激产生的NO输出[28],Src激酶也可以磷酸化Tyr83上的eNOS,从而激活eNOS[29],TMAO是否通过多位点磷酸化影响eNOS活性,使eNOS活性处于平衡状态需后期再进一步研究。除磷酸化以外,TMAO是否会影响eNOS的脂化、蛋白质直接相互作用、糖基化或是亚硝基化,有待进一步研究论证。
既往研究发现氧化应激的增加降低了eNOS活性造成NO生物利用度减而导致内皮功能障碍和老化[13-14,30]。为进一步探究腹主动脉球囊损伤模型中循环TMAO的升高是否会引起血管氧化应激的变化,本研究对损伤段动脉中ROS进行了检测。结果发现,腹主动脉球囊损伤后,模型组、TMAO增强组、TMAO抑制组ROS阳性细胞数均较正常对照组减低,其中以TMAO组减低程度最大,而以菌液治疗调节肠道菌群后,损伤段动脉ROS阳性细胞数较正常对照组差异无统计学意义,各组ROS变化趋势与eNOS Ser1177磷酸化变化趋势一致,证明TMAO降低eNOS Ser1177磷酸化水平可能通过降低ROS产生引起。但这与既往研究氧化应激的增加降低了eNOS活性结果不一致,考虑这可能与ROS的浓度有关,ROS可能作为第二信使,在高浓度时能降低eNOS活性,而低浓度时作为信号分子,可活化细胞,促进细胞增生,维持ROS在适当水平才可稳定细胞功能,因此ROS浓度差异对eNOS活性的影响需要进一步实验验证。
综上所述,TMAO可能通过eNOS-ROS途径促进内皮细胞病理性迁移增殖而加速动脉损伤后新生内膜的增生。
[1]Bjӧrkman P,Kokkonen T,Albäck A,et al.Drug-Coated versus Plain Balloon Angioplasty in Bypass Vein Grafts(the DRECOREST I-Study)[J].Ann Vasc Surg,2019,55:36-44.doi:10.1016/j.avsg.2018.04.042.
[2]Jawitz OK,Cox ML,Ranney D,et al.Outcomes following revascularization with radial artery bypass grafts:Insights from the PREVENT-IV trial[J].Am Heart J,2020,228:91-97.doi:10.1016/j.ahj.2020.08.001.
[3]Spadaccio C,Antoniades C,Nenna A,et al.Preventing treatment failures in coronary artery disease:what can we learn from the biology of in-stent restenosis,vein graft failure,and internal thoracic arteries?[J].Cardiovasc Res,2020,116(3):505-519.doi:10.1093/cvr/cvz214.
[4]Tran SM,Mohajeri MH.The Role of Gut Bacterial Metabolites in Brain Development,Aging and Disease[J].Nutrients,2021,13(3):732.doi:10.3390/nu13030732.
[5]Hou JJ,Wang X,Li Y,et al.The relationship between gut microbiota and proteolytic activity in irritable bowel syndrome[J].Microb Pathog,2021,157:104995.doi:10.1016/j.micpath.2021.104995.
[6]Aldars-García L,Marin A C,Chaparro M,et al.The Interplay between Immune System and Microbiota in Inflammatory Bowel Disease:A Narrative Review[J].Int J Mol Sci,2021,22(6):3076.doi:10.3390/ijms22063076.
[7]Wang Z,Klipfell E,Bennett B J,et al.Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease[J].Nature,2011,472(7341):57-63.doi:10.1038/nature09922.
[8]Tang WH,Hazen SL.The contributory role of gut microbiota in cardiovascular disease[J].J Clin Invest,2014,124(10):4204-4211.doi:10.1172/JCI72331.
[9]Liu Y,Dai M.Trimethylamine N-Oxide Generated by the Gut Microbiota Is Associated with Vascular Inflammation:New Insights into Atherosclerosis[J].Mediators Inflamm,2020,2020:4634172.doi:10.1155/2020/4634172.
[10]Tang WH,Wang Z,Fan Y,et al.Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-Noxide in patients with heart failure:refining the gut hypothesis[J].J Am Coll Cardiol,2014,64(18):1908-1914.doi:10.1016/j.jacc.2014.02.617.
[11]Yin J,Liao S X,He Y,et al.Dysbiosis of Gut Microbiota With Reduced Trimethylamine-N-Oxide Level in Patients With Large-Artery Atherosclerotic Stroke or Transient Ischemic Attack[J].J Am Heart Assoc,2015,4(11):e002699.doi:10.1161/JAHA.115.002699.
[12]Ufnal M,Jazwiec R,Dadlez M,et al.Trimethylamine-N-oxide:a carnitine-derived metabolite that prolongs the hypertensive effect of angio tensin II in rats[J].Can J Cardiol,2014,30(12):1700-1705.doi:10.1016/j.cjca.2014.09.010.
[13]Ke Y,Li D,Zhao M,et al.Gut flora-dependent metabolite Trimethylamine-N-oxide accelerates endothelial cell senescence and vascular aging through oxidative stress[J].Free Radic Biol Med,2018,116:88-100.doi:10.1016/j.freeradbiomed.2018.01.007.
[14]Li T,Gua C,Wu B,et al.Increased circulating trimethylamine N-oxide contributes to endothelial dysfunction in a rat model of chronic kidney disease[J].Biochem Biophys Res Commun,2018,495(2):2071-2077.doi:10.1016/j.bbrc.2017.12.069.
[15]谈路轩,李超,张振玉.欧洲《粪菌移植临床应用和操作共识报告》介绍[J].胃肠病学,2017,22(10):634-637.doi:10.3969/j.issn.1008-7125.2017.
Tan LX,Li C,Zhang ZY.Brief Introduction of European Consensus Report on Clinical Applications and Procedures of Fecal Microbiota Transplantation[J].Chinese Journal of Gastroenterology,2017,22(10):634-637.doi:10.3969/j.issn.1008-7125.2017.
[16]Wang Z,Levison B S,Hazen J E,et al.Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass sp ectrometry[J].Anal Biochem,2014,455:35-40.doi:10.1016/j.ab.2014.03.016.
[17]Luo W,Wang Y,Yang H,et al.Heme oxygenase-1 ameliorates oxidative stress-induced endothelial senescence via regulating endothelial nitric oxide synthase activation and coupling[J].Aging(Albany NY),2018,10(7):1722-1744.doi:10.18632/aging.101506.
[18]Hu A,Huang J,Li S,et al.Involvement of stromal cell-derived factor-1α(SDF-1α),stem cell factor(SCF),fractalkine(FKN)and VEGF in TSG protection against intimal hyperplasia in rat balloon injury[J].Biomed Pharmacother,2019,110:887-894.doi:10.1016/j.biopha.2018.12.030.
[19]Yang Y,Lei D,Zou H,et al.Hybrid electrospun rapamycin-loaded small-diameter decellularized vascular grafts effectively inhibit intimal hyperplasia[J].Acta Biomater,2019,97:321-332.doi:10.1016/j.actbio.2019.06.037.
[20]Ye B,Wu Z H,Tsui T Y,et al.lncRNA KCNQ1OT1 Suppresses the Inflammation and Proliferation of Vascular Smooth Muscle Cells through IκBa in Intimal Hyperplasia[J].Mol Ther Nucleic Acids,2020,20:62-72.doi:10.1016/j.omtn.2020.01.032.
[21]邬光敏,郭媛媛,朱凡,等.静脉指纹分子EphB4在移植静脉适应动脉血流环境中的调控机制[J].中国普通外科杂志,2018,27(12):1563-1569.doi:10.3978/j.issn.1005-6947.2018.12.011.
Wu GM,Guo YY,Zhu F,et al.Mechanism of venous molecular fingerprint EphB4 in regulating vein graft adaptation to the arterial hemodynamic environment[J].Chinese Journal of General Surgery,2018,27(12):1563-1569.doi:10.3978/j.issn.1005-6947.2018.12.011.
[22]Astudillo AA,Mayrovitz HN.The Gut Microbiome and Cardiovascular Disease[J].Cureus,2021,13(4):e14519.doi:10.7759/cureus.14519.
[23]Tang WHW,Li XS,Wu Y,et al.Plasma trimethylamine N-oxide(TMAO)levels predict future risk of coronary artery disease in apparen tly healthy individuals in the EPIC-Norfolk prospective population study[J].Am Heart J,2021,236:80-86.doi:10.1016/j.ahj.2021.01.020.
[24]Zheng S,Shao S,Qiao Z,et al.Clinical Parameters and Gut Microbiome Changes Before and After Surgery in Thoracic Aortic Dissection in Patients with Gastrointestinal Complications[J].Sci Rep,2017,7(1):15228.doi:10.1038/s41598-017-15079-0.
[25]Chou RH,Chen CY,Chen IC,et al.Trimethylamine N-Oxide,Circulating Endothelial Progenitor Cells,and Endothelial Function in Patient s with Stable Angina[J].Sci Rep,2019,9(1):4249.doi:10.1038/s41598-019-40638-y.
[26]Chen ML,Zhu XH,Ran L,et al.Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-mtROS Signaling Pathway[J].J Am Heart Assoc,2017,6(9):e006347.doi:10.1161/JAHA.117.006347.
[27]Bogiatzi C,Gloor G,Allen-Vercoe E,et al.Metabolic products of the intestinal microbiome and extremes of atherosclerosis[J].Atherosclerosis,2018,273:91-97.doi:10.1016/j.atherosclerosis.2018.04.015.
[28]Lin MI,Fulton D,Babbitt R,et al.Phosphorylation of threonine 497 in endothelial nitric-oxide synthase coordinates the coupling of L-a rginine metabolism to efficient nitric oxide production[J].J Biol Chem,2003,278(45):44719-44726.doi:10.1074/jbc.M302836200.
[29]Fulton D,Church J E,Ruan L,et al.Src kinase activates endothelial nitric-oxide synthase by phosphorylating Tyr-83[J].J Biol Chem,2005,280(43):35943-35952.doi:10.1074/jbc.M504606200.
[30]Li T,Chen Y,Gua C,et al.Elevated Circulating Trimethylamine N-Oxide Levels Contribute to Endothelial Dysfunction in Aged Rats through Vascular Inflammation and Oxidative Stress[J].Front Physiol,2017,8:350.doi:10.3389/fphys.2017.00350.
